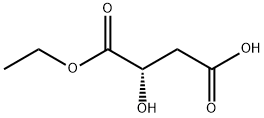
Butanedioic acid, hydroxy-, 1-ethyl ester, (S)- (9CI) synthesis
- Product Name:Butanedioic acid, hydroxy-, 1-ethyl ester, (S)- (9CI)
- CAS Number:83540-95-8
- Molecular formula:C6H10O5
- Molecular Weight:162.14
Yield:-
Reaction Conditions:
Stage #1: ethanolwith acetyl chloride at 0; for 5 h;Large scale;
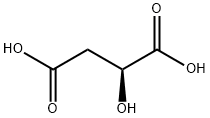
Stage #2: (S)-Malic acid at -10 - 27; for 18 h;Large scale;
Steps:
1.1 Step 1: (S)-Diethyl 2-hydroxysuccinate (5-1)
To an inerted reactor was charged ethanol (2400 kg). Agitation was started and upon cooling to 0 ± 10 °C, acetyl chloride (708 kg, 9,000 mol) was added slowly over the course of approx.5 hrs. Next, L(-)-malic acid (600 kg, 4,470 mol) was charged to the mixture while maintaining the batch between -10 to -20 °C. The mixture was then warmed to 23-27 °C and stirred for 18 hrs after which the starting material was deemed consumed by TLC. The batch was then concentrated under reduced pressure and the residue was diluted with DCM (1050 kg) and water (900 kg). After agitating for 15 min., agitation was stopped and the phases were separated. The aqueous phase was back-extracted with DCM (600 kg). The organic phases were combined and washed with 8% aqueous sodium bicarbonate (1100 kg) and 20% brine (600 kg). After drying over Na2SO4(174 kg), the batch was filtered and concentrated under reduced pressure. THF (250 kg) was charged to the residue and the solution was evaporated to dryness to afford 5-1 as yellow oil (569 kg, 66.9% yield, 93% purity) which was used without further purification.
References:
WO2022/16195,2022,A1 Location in patent:Paragraph 0105-0107