
Cyclobutanecarboxylic acid, 3-(phenylMethoxy)-, Methyl ester, trans- synthesis
- Product Name:Cyclobutanecarboxylic acid, 3-(phenylMethoxy)-, Methyl ester, trans-
- CAS Number:84182-50-3
- Molecular formula:C13H16O3
- Molecular Weight:220.26
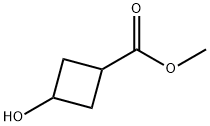
4934-99-0
89 suppliers
$21.00/1g

100-39-0
425 suppliers
$10.00/10g

84182-50-3
18 suppliers
$470.00/1g
Yield:84182-50-3 15.4%
Reaction Conditions:
Stage #1: methylester of 3-hydroxycyclobutane-1-carboxylic acidwith sodium hydride in N,N-dimethyl-formamide at 0; for 0.25 h;
Stage #2: benzyl bromide in N,N-dimethyl-formamide at 0 - 2; for 2 h;
Steps:
16.5
5) Synthesis of trans-3-hydroxy-cyclobutanecarboxylic acid methyl ester; To a solution of cis-trans mixture of 3-hydroxy-cyclobutanecarboxylic acid methyl ester (1.30 g, 10 mmol) in DMF 13 mL, NaH (50% in oil, 720 mg, 15 mmol) is added at 0° C. After stirring at 0° C. for 15 minutes, benzyl bromide (1.43 ml, 12 mmol) is added at 0° C. The mixture is stirred at room temperature for 2 hours and quenched with H2O. The solution is extracted with AcOEt. The organic layer is washed with H2O and brine, dried over MgSO4 and concentrated under reduced pressure. The residue is purified by silica gel column chromatography to give trans-3-benzyloxy-cyclobutanecarboxylic acid methyl ester (340 mg, 15.4%); TLC (hexane/AcOEt, 5:1) Rf 0.40, 1H NMR (400 MHz, chloroform-d) ppm 2.26-2.34 (m, 2H), 2.48-2.52 (m, 2H), 3.02-3.06 (m, 1H), 3.69 (s, 3H), 4.26-4.33 (m, 1H), 4.42 (s, 2H), 7.27-7.35 (m, 5H).
References:
US2009/118287,2009,A1 Location in patent:Page/Page column 72-73