
1,3,4,6-Tetra-O-acetyl-2-azido-2-deoxy-D-galactopyranose synthesis
- Product Name:1,3,4,6-Tetra-O-acetyl-2-azido-2-deoxy-D-galactopyranose
- CAS Number:84278-00-2
- Molecular formula:C14H19N3O9
- Molecular Weight:0

108-24-7
5 suppliers
$14.00/250ML

68733-26-6
79 suppliers
inquiry

84278-00-2
33 suppliers
$60.00/100mg
Yield:84278-00-2 64%
Reaction Conditions:
with pyridine;4-dimethylaminopyridine at 20;
Steps:
4.1. Synthesis of Nα-lauryl-O-(2-acetamido-2-deoxy-α-Dgalactopyranosyl)-L-threonineamide (GalNAc-Thr-C12)
General procedure: The synthetic route for the preparation of GalNAc-Thr-C12 is shownin Fig. 4. NaN3 (29.31 g) was dissolved in a mixture of 75 mL H2O and120 mL CH2Cl2; Tf2O (trifluoromethane sulfonic anhydride, 15.1 mL)was added within 5 min, and the solution was vigorously stirring on icefor 2 h. The combined organic phase was extracted with saturatedaqueous (sat. aq.) Na2CO3 solution, and the TfN3 solution was obtained.To a solution of compound 1 (D(+)-galactosamine hydrochloride, 9.92g) in 150 mL H2O, K2CO3 (9.54 g), CuSO45H2O (114.9 mg), 300 mLMeOH and the TfN3 solution were added. After stirring overnight at 25°C, the solvent was removed under reduced pressure. Next, thereactant (compound 2) was mixed with 250 mL pyridine, 200 mL aceticanhydride, and DMAP (4-dimethylaminopyridine, 562.0 mg) on ice.After stirring overnight at room temperature, 200 mL ethanol was addedon ice to stop the reaction. The solvent was removed under reducedpressure. The product was dissolved in ethyl acetate, and was washedwith 2 M HCl, H2O, sat. aq. NaHCO3 solution, and sat. aq. NaCl solution.After filtration, the solvent was removed under reduced pressure.Compound 3 was isolated by column chromatography (silica gel 60 N, 7× 17 cm, hexane/ethyl acetate = 2page10/1), with a yield of 11 g(64%).
References:
Ide, Yoshimi;Mizuno, Mamoru;Nagai, Kaori;Sakura, Ryuma;Sato, Toshinori;Takahashi, Yoshihisa;Yagi, Yuka;Yagi, Yuki;Yamamoto, Daiki [Carbohydrate Research,2022,vol. 511,art. no. 108495]
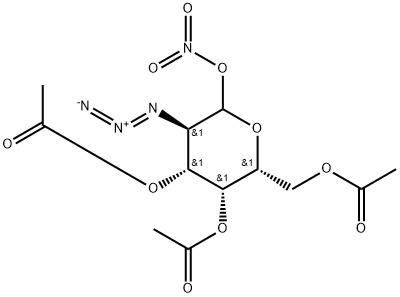
196398-25-1
3 suppliers
inquiry

84278-00-2
33 suppliers
$60.00/100mg
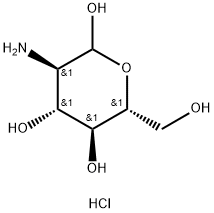
1078691-95-8
9 suppliers
inquiry

84278-00-2
33 suppliers
$60.00/100mg

68733-26-6
79 suppliers
inquiry

108-24-7
5 suppliers
$14.00/250ML

84278-00-2
33 suppliers
$60.00/100mg