
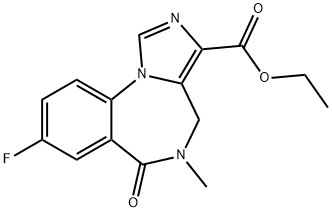
4H-IMIDAZO[1,5-A][1,4]BENZODIAZEPINE-3-CARBOXYLIC ACID, 8-FLUORO-5,6-DIHYDRO-5-METHYL-6-OXO- synthesis
- Product Name:4H-IMIDAZO[1,5-A][1,4]BENZODIAZEPINE-3-CARBOXYLIC ACID, 8-FLUORO-5,6-DIHYDRO-5-METHYL-6-OXO-
- CAS Number:84378-44-9
- Molecular formula:C13H10FN3O3
- Molecular Weight:275.24
78755-81-4
364 suppliers
$17.00/5mg
![4H-IMIDAZO[1,5-A][1,4]BENZODIAZEPINE-3-CARBOXYLIC ACID, 8-FLUORO-5,6-DIHYDRO-5-METHYL-6-OXO-](/CAS/GIF/84378-44-9.gif)
84378-44-9
75 suppliers
$80.00/5mg
Yield: 100%
Reaction Conditions:
Stage #1:flumazenil with sodium hydroxide in tetrahydrofuran;water at 20;
Stage #2: with hydrogenchloride in water
Steps:
3.1: 8-Fluoro-5-methyl-6-oxo-5,6-dihydro-4H-benzo[f]imidazo[1,5-a][1,4]diazepine-3-carboxylic acid
Flumazenil (0.5 g, 1.7 mmol) was suspended in THF (50 mL). Sodium hydroxide (10 mL of a 2M solution in water) was added and the suspended solid dissolved. The mixture was stirred at room temperature until judged complete by thin layer chromatography (TLC). The solvent was removed in vacuo and the residue dissolved in water, treated with 2M hydrochloric acid to precipitate the acid. The solid was filtered off and dried in vacuo to give a colourless solid (0.45 g, 100%). 1H NMR (CD3OD): dH 3.24 (3H. s, CH3), 4.62 (1H, br d, J = 15.4 Hz, CHaHb), 5.19 (1H, br d, J = 15.4 Hz, CHaHb), 7.62 (1H, ddd, JHF = 12.5 Hz, JHH = 7.7 and 3.1 Hz, CFCHCH), 7.79 (1H, dd, JHF = 8.9 Hz, JHH = 3.1 Hz, CCHCF), 7.87 (1H, dd, JHF = 9.1 Hz, JHH = 4.5 Hz, CHCHCF), and 9.63 (1H, s, NCHN).
References:
Jackson, Alexander;Guilbert, Benedicte B.;Plant, Stuart D.;Goggi, Julian;Battle, Mark R.;Woodcraft, John L.;Gaeta, Alessandra;Jones, Clare L.;Bouvet, Denis R.;Jones, Paul A.;O'Shea, Dennis M.;Zheng, Penny Hao;Brown, Samantha L.;Ewan, Amanda L.;Trigg, William [Bioorganic and Medicinal Chemistry Letters,2013,vol. 23,# 3,p. 821 - 826] Location in patent:supporting information