
5-(4-bromo-2-fluorophenyl)-3-methyl-1,2,4-oxadiazole synthesis
- Product Name:5-(4-bromo-2-fluorophenyl)-3-methyl-1,2,4-oxadiazole
- CAS Number:845306-17-4
- Molecular formula:C9H6BrFN2O
- Molecular Weight:257.06
![Benzamide, 4-bromo-N-[1-(dimethylamino)ethylidene]-2-fluoro-](/CAS/20210305/GIF/845306-18-5.gif)
845306-18-5
0 suppliers
inquiry

845306-17-4
12 suppliers
$358.00/500mg
Yield:-
Reaction Conditions:
with sodium hydroxide;hydroxylamine hydrochloride;acetic acid in 1,4-dioxane at 20 - 90; for 3.5 h;
Steps:
32
5-(4-Bromo-2-fluorophenyl)-3-methyl-1 ,2,4-oxadiazole (D32); 4-Bromo-2-fluorobenzoic acid (5.27g) was heated at reflux in thionyl chloride (50ml) for 4h and then allowed to cool. The mixture was evaporated in vacuo and the residue re- evaporated with DCM (2x) to give the acid chloride as a light brown oil. This was added dropwise to vigorously stirred, ice-cooled concentrated aqueous ammonia (50ml) and when addition was complete the mixture was stirred for 5min and then extracted (3x) with EtOAc. The combined organic extracts were washed with water and brine, dried (Na2SO4) and evaporated to give 4-bromo-2-fluorobenzamide as a white solid (4.72g). This material and /V,Λ/-dimethylacetamide dimethyl acetal (17ml) were heated together at 1200C for 2h. The reaction was allowed to cool to rt and the liquid evaporated in vacuo to give a brown gum which was partitioned between saturated aqueous sodium hydrogen carbonate and EtOAc. The organic extract was washed with water and brine, dried (Na2SO4) and evaporated to a gum. This was purified by chromatography (silica gel, eluant hexane/EtOAc) to give the acylamidine intermediate as a gum which solidified in vacuo (4.15g). Hydroxylamine hydrochloride (1.32g) in 1 N NaOH solution (23.5ml) was added, followed by dioxane (23.5ml) then AcOH (30ml). The reaction mixture was stirred at rt for 30min then heated at 900C for 3h. The reaction was allowed to cool to rt and poured into water. The pH was adjusted to ~9 by addition of solid NaHCO3 and the precipitated product was collected by filtration, washed on the filter with water and dried at 4O0C in vacuo to give the title compound (D32) as a greyish-brown solid (2.82g). LCMS electrospray (+ve) 257 and 259 (MH+).
References:
WO2006/40192,2006,A1 Location in patent:Page/Page column 33
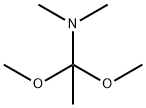
18871-66-4
210 suppliers
$11.00/1g

292621-45-5
81 suppliers
$35.00/250mg

845306-17-4
12 suppliers
$358.00/500mg