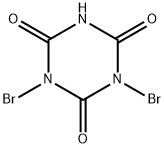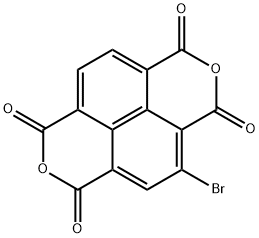
2-bromo-1,4,5,8-naphthalenetetracarboxylic acid dianhydride synthesis
- Product Name:2-bromo-1,4,5,8-naphthalenetetracarboxylic acid dianhydride
- CAS Number:846-20-8
- Molecular formula:C14H3BrO6
- Molecular Weight:347.07
Yield:846-20-8 71%
Reaction Conditions:
with sulfuric acid for 12 h;Heating;Inert atmosphere;
Steps:
1 Synthesis of compound 4:
According to the above reaction formula, 1,3-dibromo-1,3,5-triazine-2,4,6-trione (compound 3) (5.56 g, 19.4 mmol) was dissolved in 30 mL of 98 under the protection of nitrogen. In concentrated sulfuric acid, 1,4,5,8-naphthalenetetracarboxylic dianhydride(purchased from Shenzhen Ruixun Optoelectronic Material Technology Co., Ltd., 5.5 g, 20.51 mmol) was dissolved in 30 mL of concentrated sulfuric acid.Mix the two at 130 ° C and stir for 12 hours.After the reaction is completed, cool the mixture to 0 ° C.200 mL of deionized water was added to quench the reaction,Filter the crude product and rinse with deionized water and ethanol.Drying gave 5.07 g of orange-yellow solid product (Compound 3-i) in a yield of 71%. Under nitrogen protection,The excess product 3-i (4.0 g, 11.53 mmol) was weighed and dissolved in glacial acetic acid (450 mL), and then 2-ethylhexylamine (7.2 g, 55.66 mmol) was slowly added dropwise. After the dropwise addition was completed, the temperature was raised to 130 ° C and stirred for 1 hour. After the reaction is complete,After cooling to room temperature, the reaction solution was slowly poured into 200 ml of stirred ice water, and the precipitate was filtered, washed with water to remove the attached glacial acetic acid, and then extracted with chloroform.The crude product was recrystallized from a mixed solvent of ethyl acetate / ethanol to obtain 3.63 g of an orange-yellow solid product (Compound 4) with a yield of 68% and a mass spectral molecular weight of 569.6 g / mol.
References:
CN110407860,2019,A Location in patent:Paragraph 0060; 0064-0066
81-30-1
240 suppliers
$6.00/5g

846-20-8
21 suppliers
$246.00/1G

81-30-1
240 suppliers
$6.00/5g

846-20-8
21 suppliers
$246.00/1G
![4,9-DibroMoisochroMeno[6,5,4-def]isochroMene-1,3,6,8-tetraone](/CAS/GIF/83204-68-6.gif)
83204-68-6
78 suppliers
$45.00/10mg
![4,5,9,10-Tetrabromoisochromeno[6,5,4-def]isochromene-1,3,6,8- tetraone](/CAS/20180906/GIF/299962-88-2.gif)
299962-88-2
33 suppliers
inquiry