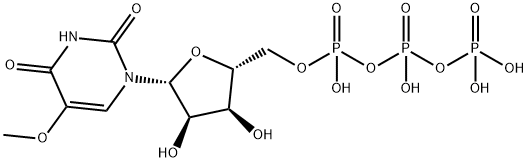
Uridine 5'-(tetrahydrogen triphosphate), 5-methoxy- synthesis
- Product Name:Uridine 5'-(tetrahydrogen triphosphate), 5-methoxy-
- CAS Number:847649-65-4
- Molecular formula:C10H17N2O16P3
- Molecular Weight:514.17
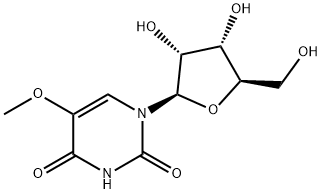
35542-01-9
78 suppliers
$65.00/10mg

847649-65-4
12 suppliers
inquiry
Yield:-
Reaction Conditions:
Stage #1: 5-methoxycarbonylmethyl-2'-O-methyl-uridinewith trimethyl phosphite;N,N,N',N'-tetramethyl-1,8-diaminonaphthalene at 0; for 0.166667 h;
Stage #2: with trichlorophosphate for 2 h;Inert atmosphere;
Stage #3: with tributyl-amine;bis(tributylammonium) pyrophosphate in N,N-dimethyl-formamide; for 0.25 h;
Steps:
32 Example 32. Synthesis of 5-methoxy uridine (compound 15) and 5-methoxy UTP (NTP of said compound)
A solution of 5-methoxy uridine (compound 15) (69.0 mg, 0.25 mmol, plus heat to make itsoluble) was added to proton sponge (80.36 mg, 0.375 mmol, 1.50 equiv.) in 0.7 mLtrimethylphosphate (TMP) and was stirred for 10 minutes at 0C. Phosphorous oxychloride (POOl3)(46.7 ul, 0.50 mmol, 2.0 equiv.) was added dropwise to the solution before being kept stirring for 2hours under N2 atmosphere. After 2 hours the solution was reacted with a mixture ofbistributylammonium pyrophosphate (TBAPP or (n-Bu3NH)2H2P207) (894.60 mg, 1.63 mmol, 6.50 equiv.) and tributylamine (243.0 ul, 1.00 mmol, 4.0 equiv.) in 2.0 ml of dimethylformamide. After approximately 15 minutes, the reaction was quenched with 17.0 ml of 0.2M triethylammonium bicarbonate (TEAB) and the clear solution was stirred at room temperature for an hour. The reactionmixture was lyophilized overnight and the crude reaction mixture was purified by HPLC (Shimadzu, Kyoto Japan, Phenomenex 018 preparative column, 250 x 21 .20 mm, 10.0 micron; gradient: 100 % A for 3.0 mm, then 1% B/mm, A = 100 mM TEAB buffer, B = ACN; flow rate: 10.0 mL/min; retention time: 16.57-1 7.51 mm). Fractions containing the desired compound were pooled and lyophilized to produce the NTP of compound 15. The triphosphorylation reactions were carried out in a two-neckflask flame-dried under N2 atmosphere. Nucleosides and the protein sponge were dried over P205 under vacuum overnight prior to use. The formation of monophosphates was monitored by LOMS.
References:
WO2015/196130,2015,A2 Location in patent:Page/Page column 598