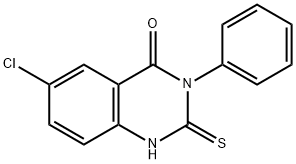
6-CHLORO-3-PHENYL-2-THIOXO-2,3-DIHYDRO-4(1H)-QUINAZOLINONE synthesis
- Product Name:6-CHLORO-3-PHENYL-2-THIOXO-2,3-DIHYDRO-4(1H)-QUINAZOLINONE
- CAS Number:84772-27-0
- Molecular formula:C14H9ClN2OS
- Molecular Weight:288.75
Yield:84772-27-0 38%
Reaction Conditions:
with triethylamine in ethanol;Reflux;
Steps:
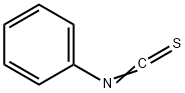
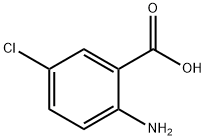
6-Chloro-or 6,7-dimethoxy-2-mercapto-3-(phenyl or benzyl)quinazolin-4(3H)-ones (14,16-17)
General procedure: A mixture of the anthranilic acid derivatives 10, 11 (0.001 mol), the isothiocyanates 12, 13 (0.001mol) andtriethylamine (2 ml), in absolute ethanol (50 ml), was stirred and heated underreflux for 3-4 hours. The precipitate formed was filtered, dried and trituratedwith petroleum ether (40°-60°) to remove excess isothiocyanate and recrystallizedfrom ethanol to afford 14-17 as solid products (Table1)
References:
Al-Salem, Huda S.A.;Hegazy, Gehan H.;El-Taher, Kamal E.H.;El-Messery, Shahenda M.;Al-Obaid, Abdulrahman M.;El-Subbagh, Hussein I. [Bioorganic and Medicinal Chemistry Letters,2015,vol. 25,# 7,p. 1490 - 1499] Location in patent:supporting information