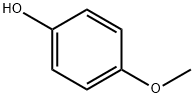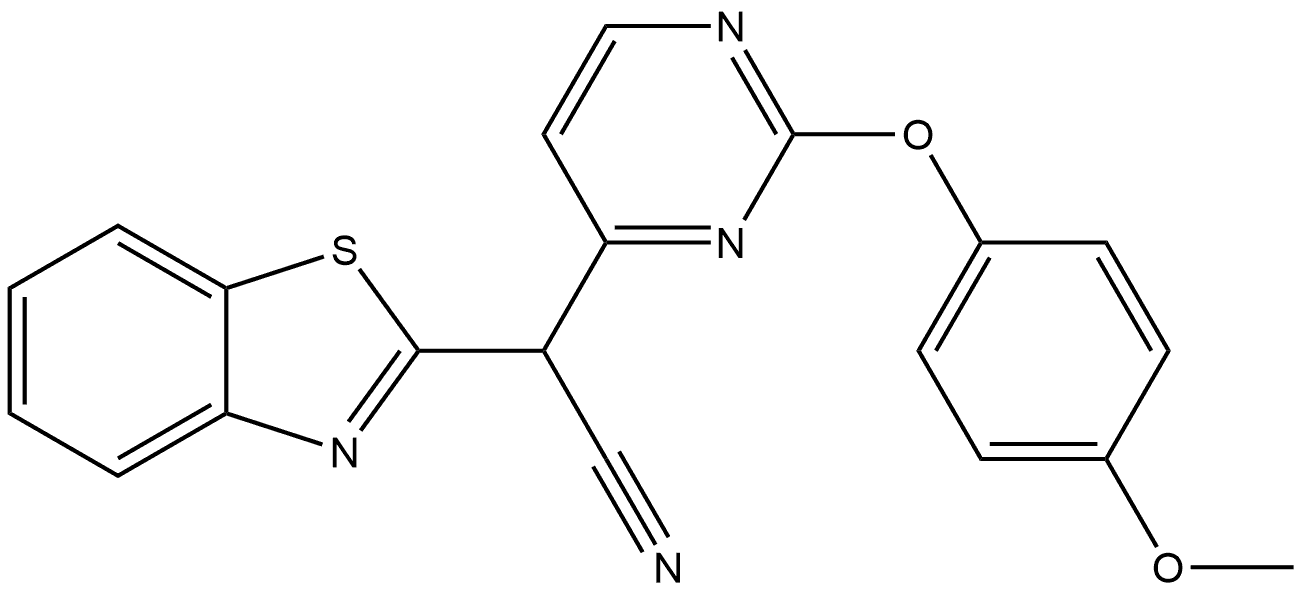
2-Benzothiazoleacetonitrile, α-[2-(4-methoxyphenoxy)-4-pyrimidinyl]- synthesis
- Product Name:2-Benzothiazoleacetonitrile, α-[2-(4-methoxyphenoxy)-4-pyrimidinyl]-
- CAS Number:848344-39-8
- Molecular formula:C20H14N4O2S
- Molecular Weight:374.42
Yield:-
Reaction Conditions:
with caesium carbonate in ethanol;dimethyl sulfoxide;
Steps:
4 Preparation of 1,3-benzothiazol-2-yl[2-(4-methoxyphenoxy)pyrimidin-4-yl]acetonitrile (96)
Example 4 Preparation of 1,3-benzothiazol-2-yl[2-(4-methoxyphenoxy)pyrimidin-4-yl]acetonitrile (96) To a solution of 1 (0.300 g, 1.05 mmol) in DMSO (7 ml) were added 4-methoxyphenol (0.261 g, 2.1 mmol) and cesium carbonate (1.7 g, 5.25 mmol) and the suspension was shaken at 100° C. for 8 days. After cooling to r.t., the suspension was poured onto ice/water and the product was extracted with AcOEt. The organic phases were washed with water then brine, dried over MgSO4 and concentrated to dryness. The residue was triturated in hot EtOH then filtered off and dried under vacuum at 50° C. overnight, affording 202 mg (51%) of the title compound. MS: 375.0 (M+1); HPLC (Conditions c, max plot): 99%/o, rt. 5.21 min 1H NMR (DMSO-d6) δ8.16 (br d, 1H), 7.60 (d, J=8.29 Hz, 1H), 7.45-7.37 (m, 2H), 7.28-7.23 (m, 3H), 7.11-7.08 (m, 2H), 6.83 (br d, 1H), 3.86 (s, 3H).
References:
US2003/162794,2003,A1
![2-(benzo[d]thiazol-2-yl)-2-(2-chloropyriMidin-4-yl)acetonitrile](/CAS/GIF/345986-38-1.gif)