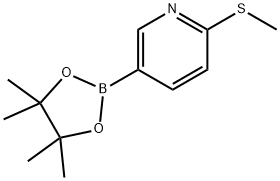
2-METHYLSULFANYL-5-(4,4,5,5-TETRAMETHYL-[1,3,2]-DIOXABOROLAN-2-YL)PYRIDINE synthesis
- Product Name:2-METHYLSULFANYL-5-(4,4,5,5-TETRAMETHYL-[1,3,2]-DIOXABOROLAN-2-YL)PYRIDINE
- CAS Number:849934-89-0
- Molecular formula:C12H18BNO2S
- Molecular Weight:251.15
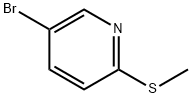
51933-78-9
109 suppliers
$15.00/250mg

73183-34-3
588 suppliers
$6.00/5g
![2-METHYLSULFANYL-5-(4,4,5,5-TETRAMETHYL-[1,3,2]-DIOXABOROLAN-2-YL)PYRIDINE](/CAS/GIF/849934-89-0.gif)
849934-89-0
42 suppliers
$452.00/5g
Yield:849934-89-0 97%
Reaction Conditions:
with nitrogen;potassium acetate;triethylamine;palladium in hexane;dichloromethane;water;dimethyl sulfoxide;ethyl acetate;
Steps:
9 Compounds of Formula (KB)
Preparation 9 Compounds of Formula (KB) Palladium catalyst ([1,1'-bis(diphenylphosphino)ferrocene]dichloropalladium (II) complex with dichloromethane (1:1), 167 mg, 0.21 mmol), potassium acetate (1.81 g, 18.5 mmol, Aldrich), and bis(pinacolato)diboron (1.56 g, 6.1 mmol) were placed into a vial and degassed with stream of nitrogen for 20 min. In a separate vial, 5-bromo-2-methylsulfanylpyridine (836 mg, 4.1 mmol) was dissolved in 8 ml anhydrous DMSO and degassed with stream of nitrogen for 20 min. The DMSO solution of 5-bromo-2-methylsulfanylpyridine was added to the "catalyst" vial, and then heated at 80° C. overnight. After cooling to ambient temperature, water and ethyl acetate were added to the reaction mixture. The aqueous layer was extracted with ethyl acetate. The combined organic layers were washed with brine, dried over sodium sulfate, and concentrated in vacuo. The crude product was purified by column chromatography on silica gel (10→30% EtOAC/Hexane, 0.25% Et3N in hexane) to give 2-methylsulfanyl-5-(4,4,5,5-tetramethyl-[1,3,2]dioxaborolan-2-yl)pyridine as a colorless oil (1.00 g, 97% yield). 1H-NMR (400 MHz, CDCl3): δ 1.35 (s, 12H), 2.58 (s, 3H), 7.16 (dd, 1H, J=1.0, J=8.0), 7.83 (dd, 1H, J=1.8, J=8.0), 8.50 (dd, 1H, J=1.7, J=1.0).
References:
US2005/80111,2005,A1

51933-78-9
109 suppliers
$15.00/250mg

5419-55-6
386 suppliers
$12.00/5g
![2-METHYLSULFANYL-5-(4,4,5,5-TETRAMETHYL-[1,3,2]-DIOXABOROLAN-2-YL)PYRIDINE](/CAS/GIF/849934-89-0.gif)
849934-89-0
42 suppliers
$452.00/5g
123-91-1
699 suppliers
$20.00/25g

51933-78-9
109 suppliers
$15.00/250mg
![2-METHYLSULFANYL-5-(4,4,5,5-TETRAMETHYL-[1,3,2]-DIOXABOROLAN-2-YL)PYRIDINE](/CAS/GIF/849934-89-0.gif)
849934-89-0
42 suppliers
$452.00/5g
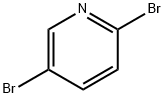
624-28-2
729 suppliers
$5.00/5g
![2-METHYLSULFANYL-5-(4,4,5,5-TETRAMETHYL-[1,3,2]-DIOXABOROLAN-2-YL)PYRIDINE](/CAS/GIF/849934-89-0.gif)
849934-89-0
42 suppliers
$452.00/5g
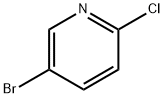
53939-30-3
471 suppliers
$5.00/1g
![2-METHYLSULFANYL-5-(4,4,5,5-TETRAMETHYL-[1,3,2]-DIOXABOROLAN-2-YL)PYRIDINE](/CAS/GIF/849934-89-0.gif)
849934-89-0
42 suppliers
$452.00/5g