
850222-41-2 synthesis
- Product Name:850222-41-2
- CAS Number:850222-41-2
- Molecular formula:C31H33NO10
- Molecular Weight:579.6
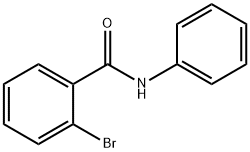
10282-57-2
13 suppliers
inquiry
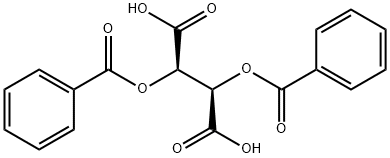
2743-38-6
541 suppliers
$10.00/5g

1217854-15-3
4 suppliers
inquiry

850222-41-2
12 suppliers
inquiry
Yield:850222-41-2 80.5%
Reaction Conditions:
in acetone at 35 - 40; for 27 h;Purification / work up;Resolution of racemate;
Steps:
a''.1.a
1. a. Preparation of (S)-3-(dimethylamino)-1-(3-methoxyphenyl)-2- methylpropan-1-one (2R,3R)-O,O'-dibenzoyltartrate in acetone; (2R,3R)-O,O'-Dibenzoyl tartaric acid monohydrate (189.1 g, 0.5 mol) was dissolved in acetone (550 mL) in a 2 L reaction plant equipped with a mechanical stirrer, temperature measuring equipment and an oil bath and 3-(dimethylamino)-1-(3- methoxyphenyl)-2-methylpropan-1-one (110.6 g, 0.5 mol) was added. The reaction mixture was heated to 35 0C to 40 0C for 27 hours and allowed to cool to 25 0C. The suspension was siphoned off and (S)-3-(dimethylamino)-1-(3-methoxyphenyl)-2- methylpropan-1-one (2R,3R)-O,O'-dibenzoyltartrate was obtained as a colorless solid (233.2 g, 80.5 %, ee 96.9 %, ee = enantiomeric excess).
References:
WO2008/12047,2008,A1 Location in patent:Page/Page column 19
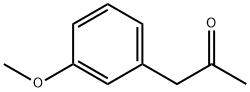
3027-13-2
145 suppliers
$21.00/250mg

850222-41-2
12 suppliers
inquiry