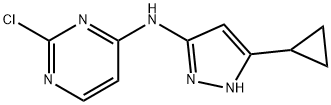
2-chloro-N-(5-cyclopropyl-1H-pyrazol-3-yl)pyriMidin-4-aMine synthesis
- Product Name:2-chloro-N-(5-cyclopropyl-1H-pyrazol-3-yl)pyriMidin-4-aMine
- CAS Number:851435-00-2
- Molecular formula:C10H10ClN5
- Molecular Weight:235.67
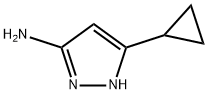
175137-46-9
192 suppliers
$16.00/250mg
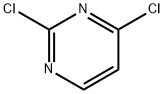
3934-20-1
707 suppliers
$9.00/1g

851435-00-2
19 suppliers
inquiry
Yield:851435-00-2 81%
Reaction Conditions:
with N-ethyl-N,N-diisopropylamine in dimethyl sulfoxide at 20 - 60; for 16 h;
Steps:
4 Referential Example 4 2-Chloro-N-(3-cyclopropyl-lH-pyrazol-5-yl)pyrimidin-4-amine (53)
Referential Example 4 2-Chloro-N-(3-cyclopropyl-lH-pyrazol-5-yl)pyrimidin-4-amine (53) A dried 5-L, three-neck round bottom flask fitted with an overhead stirrer and reflux condenser was charged with a solution of 2,4-dichloropyrimidine (250 g, 1.678 mol) in anhydrous DMSO (2300 mL). 3- Cyclopropyl-lH-pyrazol-5-amine (227.4 g, 1.8458 mol) and DIPEA (438 mL, 2.517 mol) were added sequentially at RT. The resulting solution was stirred at 60°C for 16 h, cooled to RT, and poured into ice water. The precipitated yellow solid was collected by vacuum filtration, and washed well with water, 1.5 N HQ (3 x 1 L), and finally rinsed of water (4 x 500 mL). The precipitate was dried by air suction overnight to give 320 g (81 %) of 53 as yellow solid: 1H NMR (400 MHz, DMSO-d6) δ 12.19 (s, 1H), 10.29 (s, 1H), 8.15 (s, 1H), 7.0 (br s, 1H), 6.0 (br s, 1H), 1.85-1.92 (m, 1H), 0.91-0.95 (m, 2H), 0.7 (m, 2H); MS (ESI+) m/z = 236 [M +1]+.
References:
WO2013/26914,2013,A1 Location in patent:Page/Page column 92

3934-20-1
707 suppliers
$9.00/1g

175137-46-9
192 suppliers
$16.00/250mg

851435-00-2
19 suppliers
inquiry