
2(1H)-Isoquinolinecarboxylic acid, 5,7-dichloro-3,4-dihydro-6-hydroxy-, 1,1-dimethylethyl ester synthesis
- Product Name:2(1H)-Isoquinolinecarboxylic acid, 5,7-dichloro-3,4-dihydro-6-hydroxy-, 1,1-dimethylethyl ester
- CAS Number:851784-76-4
- Molecular formula:C14H17Cl2NO3
- Molecular Weight:318.2

24424-99-5
824 suppliers
$13.50/25G
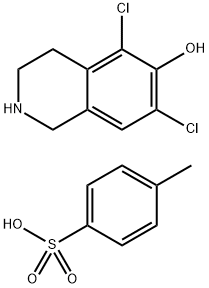
1609545-55-2
8 suppliers
inquiry

851784-76-4
14 suppliers
inquiry
Yield:851784-76-4 95%
Reaction Conditions:
with sodium carbonate in methanol at 20;
Steps:
1B Example 1B
[00119] To 1,2,3,4-tetrahydro-6-hydroxy-isoqinoline in acetonitrile was added ptoluenesulfonic acid and N-chlorosuccinimide. The suspension was cooled to ambient temperature, and the product isolated by filtration for a yield of approximately 61% with purity greater than 95%. The isolated TsOH salt was recrystallized until purity was greater than 99.7%. To one equivalent of the TsOH salt suspended in methanol was added 2M sodium carbonate(0.55 eq.) and 1.2 eq. of Boc anhydride. The suspension was stirred at room temperature overnight. The reaction was monitored by HPLC. Upon completion, the mixture was cooled to below 10 °C, water was added, and the Boc-protected dichloro compound was isolated by filtraton. The product was washed and dried at 40 °C for a yield of 95% and purity of >97%. The Boc-protected dichloro compound was suspended in dichloromethane (10 volumes) and pyridine(5 volumes) was added. The mixture was cooled to below 2 °C, and triflic anhydride (1.25 eq) was added. The mixture was stirred at 0-2 °C for 10 minutes, and then poured into 10 volumes of 6% aqueous sodium hydrogen carbonate solution. After washing with dichloromethane, the organic phases were combined and dried over magnesium sulphate. Following purification, the product (Compound 4’) was obtained in 90% yield and >98% purity. Compound 4’ was dissolved in dimethylformamide and methanol at room temperature. Diisopropylamine (4 eq) was added. Under CO atmosphere, 1,3-bis(diphenylphosphino)propane (0.1 eq) and palladium acetate (0.1 eq) was added. The reaction was heated to reflux, and monitored by HPLC. Uponnear completion, the mixture was cooled to ambient temperature. Workup with water, ethyl aceate, and brine yielded Compound 4”, which was used without further purification. Compound 4” was dissolved in methanol and 2.4 M sodium hydroxide (10 volumes each) and refluxed. The mixture was cooled to ambient temperature, and toluene was added. Following aqueous workup, the pH was adjusted to 2.3 with 3M hydrochloric acid, and crude product was isolated byfiltration in 53% yield with greater than 80% purity.
References:
WO2014/18748,2014,A1 Location in patent:Paragraph 00118; 00119

50-00-0
851 suppliers
$10.00/25g

24424-99-5
824 suppliers
$13.50/25G

2039-67-0
241 suppliers
$17.46/5G

851784-76-4
14 suppliers
inquiry

876108-97-3
4 suppliers
inquiry

24424-99-5
824 suppliers
$13.50/25G

851784-76-4
14 suppliers
inquiry

14446-24-3
189 suppliers
$23.00/250mg

851784-76-4
14 suppliers
inquiry