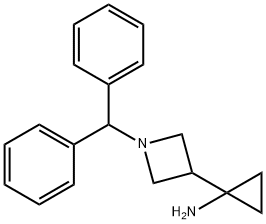
1-[1-(Diphenylmethyl)-3-azetidinyl]-cyclopropanamine synthesis
- Product Name:1-[1-(Diphenylmethyl)-3-azetidinyl]-cyclopropanamine
- CAS Number:852655-67-5
- Molecular formula:C19H22N2
- Molecular Weight:278.39
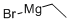
925-90-6
345 suppliers
$12.00/5ml
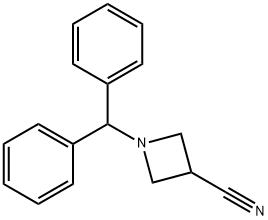
36476-86-5
185 suppliers
$6.00/1g
![1-[1-(Diphenylmethyl)-3-azetidinyl]-cyclopropanamine](/CAS/GIF/852655-67-5.gif)
852655-67-5
14 suppliers
$512.00/1g
Yield:852655-67-5 44%
Reaction Conditions:
Stage #1: ethylmagnesium bromide;1-(1,1-diphenylmethyl)azetidine-3-carbonitrilewith titanium(IV) isopropylate in tetrahydrofuran at 20; for 0.5 h;
Stage #2: with boron trifluoride diethyl etherate in tetrahydrofuran; for 0.5 h;
Steps:
B.2; A.2.A
To a solution of 1-benzhydryl-azetidine-3-carbonitrile (10 g) in THF (200 mL) were added successively at room temperature titanium isopropoxide (Ti (OiPr)4) (1 equivalent) and ethylmagnesium bromide (2.2 equivalents). The resulting reaction mixture was stirred for 30 minutes. Borontrifluoride diethyl etherate(BF30Et2) (2 equivalents) was then added. Stirring was continued for a period of 30 minutes. A solution of 10% sodium hydroxide was added, and the mixture was extracted three times with ethyl acetate (EtOAc). The combined ethyl acetate layers were dried overNa2SO4, and concentrated. The crude material was purified by chromatography (EtOAc to 7: 3 EtOAc: EtOH) to yield the title compound as a yellow solid (4.96 g, 44 % yield). MS (APCI+): m/z 279(M+H) +.
References:
WO2005/49602,2005,A1 Location in patent:Page/Page column 80; 81; 106; 107