4-Pyrimidinamine, 2-chloro-5-methyl-N-[(1S)-1-(3-pyridinyl)butyl]- synthesis
- Product Name:4-Pyrimidinamine, 2-chloro-5-methyl-N-[(1S)-1-(3-pyridinyl)butyl]-
- CAS Number:854074-39-8
- Molecular formula:C14H17ClN4
- Molecular Weight:276.76
Yield:-
Reaction Conditions:
with N-ethyl-N,N-diisopropylamine in ethanol; for 24 h;Heating / reflux;
Steps:
17
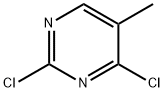
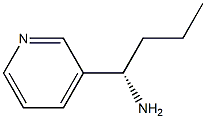
EXAMPLE 17 2-Chloro-5-methyl-N-[(1S)-1-pyridin-3-ylbutyl]pyrimidin-4-amine To a solution of (lS)-l-pyridin-3-ylbutan-1-amine (600 mg, 4.0 mmol) in ethanol (20 mL) was added 2, 4-dichloro-5-methylpyrimidine (717 mg, 4.4 mmol) and diisopropylethylamine (1.4 mL, 8mmol). The solution was heated at reflux for 24h after which time the solvent was removed under reduced pressure. The residue was dissolved in ethyl acetate (30 mL) and washed with H20 (2 x 15 mL), brine (15 mL) and dried (Na2SO4). The residue remaining after concentration in vacuo was chromatographed using ethyl acetate-hexanes (9: 1) as eluant to separate the desired product (370 mg). lH-n. m. r. (CDC13) 8 0.96 (t, 3H, J = 7. 2 Hz, CH3), 1. 23-1.52 (m, 2H, CH2), 1.85-1. 99 (m, 2H, CH2), 2.03 (d, 3H, J= 0.8 Hz, Ar-Me), 5.03 (br d, 1H,/= 7.2 Hz, NH), 5.22-5. 33 (m, 1H, CH), 7.24-7. 30 (m, 1H, ArH), 7.65-7. 71 (m, 1H, ArH), 7.81 (d, 1H,/= 0.8 Hz, ArH), 8.51 (dd, J=5. 6,1. 4 Hz, 1H, ArH), 8.64 (d, 1H, 1. 8 Hz, ArH).
References:
WO2005/54199,2005,A1 Location in patent:Page/Page column 47