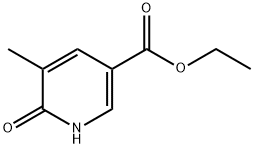
Ethyl 5-methyl-6-oxo-1,6-dihydropyridine-3-carboxylate synthesis
- Product Name:Ethyl 5-methyl-6-oxo-1,6-dihydropyridine-3-carboxylate
- CAS Number:85614-89-7
- Molecular formula:C9H11NO3
- Molecular Weight:181.19
Yield:85614-89-7 78.5%
Reaction Conditions:
Stage #1: propionyl chloride;N-tert-butyldimethylsilylisopropyl formimidatewith triethylamine in toluene at 20; for 2 h;
Stage #2: propynoic acid ethyl ester in toluene at 85; for 70 h;
Steps:
9.1.ii
ii) 6-Hydroxy-5-methyl-nicotinic acid ethyl ester At RT, a solution of propionyl chloride (1.55 mL, 17.4 mmol) in 3.5 mL of toluene was added drop-wise to a solution of N-tert-butyldimethylsilyl isopropyl formimidate (3.51 g, 17.4 mmol) and triethylamine (12.2 mL, 87 mmol) in 10 mL of toluene. The resulting mixture was stirred at RT for 2 hours and then 10 mL of hexanes was added. The precipitate was removed by filtration and washed with hexanes (3 x 5 mL). The solution was concentrated in vacuo to afford a clear oil. This oil was solubilised in toluene (15 mL) and ethyl propiolate (1.2 mL, 11.6 mmol) was added. The resulting mixture was stirred at 85°C for 70 hours. The mixture was concentrated in vacuo and then diluted with HCI 2N. The aqueous phase was extracted with DCM. The organic phases were combined, dried over sodium sulfate and concentrated in vacuo to afford a crude yellow paste (3.5 g). The crude product was purified by flash chromatography over silica gel using Hexanes/EtOAc (75/25 to 0/100) as solvent gradient to afford 6-hydroxy-5-methyl-nicotinic acid ethyl ester (1.65 g, 78.5%) as a yellow powder. (ES- MS: m/z 182.1 [M+H]+, tR 3.28 min (system 2)).
References:
WO2007/71358,2007,A1 Location in patent:Page/Page column 59
![Methanimidic acid, N-[(1,1-dimethylethyl)dimethylsilyl]-, 1-methylethyl ester](/CAS/20210305/GIF/172095-72-6.gif)
