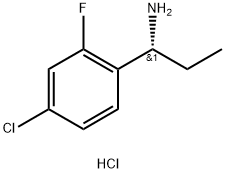
(R)-1-(4-Chloro-2-fluoro-phenyl)-propylamine hydrochloride synthesis
- Product Name:(R)-1-(4-Chloro-2-fluoro-phenyl)-propylamine hydrochloride
- CAS Number:856563-04-7
- Molecular formula:C9H12Cl2FN
- Molecular Weight:224.1
![2-Propanesulfinamide, N-[(1R)-1-(4-chloro-2-fluorophenyl)propyl]-2-methyl-, [S(R)]-](/CAS/20210305/GIF/1433401-48-9.gif)
1433401-48-9
0 suppliers
inquiry

856563-04-7
2 suppliers
inquiry
Yield:856563-04-7 99%
Reaction Conditions:
with hydrogenchloride in 1,4-dioxane;ethyl acetate at 20; for 0.5 h;Inert atmosphere;
Steps:
2 Intermediate 2 Step 2
Step 2 To a solution of (R)-(+)-2-methyl-propane-2-sulfinic acid [(R)-1-(4-chloro-2-fluoro- phenyl)-propyl]-amide (19.9 g, 68.2 mmol, 1.0 eq) in EtOAc (500 ml) was added 2.1 M HCI in dioxane (69 ml, 137.1 mmol, 2.0 eq) slowly. The reaction was stirred at room temperature under N2 for 30 min. The solvents were removed in vacuo and the crude material slurried in 3:1 heptane: Et20 (200 ml) for 20 min then filtered and the cake washed with heptanes (2 x 50 ml). The cake was dried in an oven at 35 °C under vacuum for 30 min to give (R)-1-(4-chloro-2- fluoro-phenyl)-propylamine hydrochloride (19.6 g, 1H NMR >95% excluding solvents, 77% active, 67.7 mmol, 99% yield). 1H NMR (270 MHz, DMSO-d6): 8.81 (3H, s), 7.77 (1 H, t), 7.52 (1H, dd), 7.41 (1H, dd), 4.33 (1H, q), 2.08-1.76 (2H, m), 0.76 (3H, t).
References:
WO2013/64543,2013,A1 Location in patent:Page/Page column 106-107
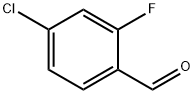
61072-56-8
290 suppliers
$14.00/5g

856563-04-7
2 suppliers
inquiry
![2-Propanesulfinamide, N-[(4-chloro-2-fluorophenyl)methylene]-2-methyl-, [N(E),S(R)]-](/CAS/20211123/GIF/926664-60-0.gif)
926664-60-0
0 suppliers
inquiry

856563-04-7
2 suppliers
inquiry