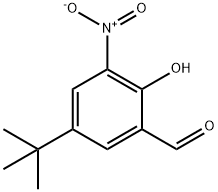
Benzaldehyde, 5-(1,1-dimethylethyl)-2-hydroxy-3-nitro- synthesis
- Product Name:Benzaldehyde, 5-(1,1-dimethylethyl)-2-hydroxy-3-nitro-
- CAS Number:85943-75-5
- Molecular formula:C11H13NO4
- Molecular Weight:223.23
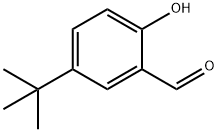
2725-53-3
142 suppliers
$20.00/1g

85943-75-5
7 suppliers
inquiry
Yield:85943-75-5 99%
Reaction Conditions:
with (NO)+18-crown-6-H(NO3)2 in ethyl acetate at 20; for 5 h;
Steps:
8 Synthesis of 3-Amino-5-tert-butyl-2-methoxy-benzonitrile
To a solution of 5-tert-butyl-2-hydroxybenzaldehyde (1.25 g, 7.0 mmol) in ethyl acetate (15 mL) was added (NO)+18-crown-6-H(NO3)2 (2.64 g, 6.3 mmol). The solution turned yellow and was stirred at room temperature for 5 h. The solvent was evaporated, giving a residue which was taken up in ether. The ether was washed 4× with saturated NH4Cl, dried over Na2SO4, filtered, and concentrated in vacuo to give 5-tert-butyl-2-hydroxyl-3-nitro-benzaldehyde (1.56 g, 99%) as a yellow solid. The above aldehyde (1.4 g, 6.3 mmol), hydroxylamine hydrochloride (0.438 g, 6.3 mmol), and sodium formate (0.771 g, 11.3 mmol) in formic acid (20 mL) were heated overnight, at reflux, then cooled to room temperature. The reaction was diluted with water, and the resulting precipitate was filtered. The solid was taken up in ether, dried over Na2SO4, filtered and concentrated in vacuo to yield 5-tert-butyl-2-hydroxyl-3-nitro-benzonitrile (1.21 g, 87%) as a yellow solid. The above nitrile (0.5 g, 2.27 mmol) was taken up in 1:9 methanol/acetonitrile (20 mL). N,N-diisopropylethylamine (1.1 mL, 6.35 mmol) was added dropwise followed by (trimethylsilyl)diazomethane (3.2 mL, 6.35 mmol). The reaction was stirred until the bubbling stopped (20 min) and the reaction was quenched with water. The water was extracted 3× with methylene chloride, dried over Na2SO4, filtered, and concentrated in vacuo to give 5-tert-butyl-2-methoxy-3-nitro-benzonitrile (0.531 g, 99%) as a yellow solid. The above benzonitrile (100 mg, 0.427 mmol) was dissolved in 1:1 ethyl acetate/methanol (10 mL) in a nitrogen-flushed flask. Ammonium formate (270 mg, 4.27 mmol) and palladium on carbon (30 mg, 10% wet) were added and the mixture was heated to reflux for 30 min. The reaction was cooled to room temperature and filtered through a pad of diatomaceous earth, eluting with ethyl acetate. The ethyl acetate was evaporated under vacuum. The resulting residue was purified by chromatography on silica gel (1:1 ethyl acetate/hexanes) to afford the title compound, (67 mg, 77%) as a colorless oil.
References:
US2005/245536,2005,A1 Location in patent:Page/Page column 25-26

98-54-4
406 suppliers
$16.00/25g

85943-75-5
7 suppliers
inquiry