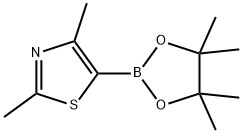
2,4-DIMETHYL-5-(4,4,5,5-TETRAMETHYL-1,3,2-DIOXABOROLAN-2-YL)-1,3-THIAZOLE synthesis
- Product Name:2,4-DIMETHYL-5-(4,4,5,5-TETRAMETHYL-1,3,2-DIOXABOROLAN-2-YL)-1,3-THIAZOLE
- CAS Number:859833-13-9
- Molecular formula:C11H18BNO2S
- Molecular Weight:239.14
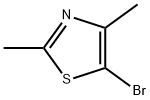
28599-52-2
97 suppliers
$27.57/250mgs:
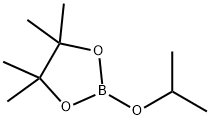
61676-62-8
313 suppliers
$11.19/5G

859833-13-9
53 suppliers
$50.00/50mg
Yield:859833-13-9 26%
Reaction Conditions:
Stage #1: 5-bromo-2,4-dimethyl-1,3-thiazolewith magnesium in tetrahydrofuran;ethylene dibromide at 75; for 5 h;
Stage #2: 2-Isopropoxy-4,4,5,5-tetramethyl-1,3,2-dioxaborolane in tetrahydrofuran;ethylene dibromide at 0 - 20; for 20 h;
Steps:
1 Production of 2,4 -Dim at hyl-5-(4, 45, 5-tetramethyl-1, 3, 2-dioxaboroIan-2-yfltliazo1e (9)
A solution of 5-bromo-2,4-dimethylthiazole (8) (5.00 g. 26.0 mmo) and 1 .2- dibromoethane (0.24 91 1.3 mmol) in THF (20 mL) was added dropwise to a flask containing magnesium turnings (0.65 g, 26.8 rnmol) over a one hour period. The reaction mixture was heated to 75 CC for 4 hours, cooled to ambient temperature then transferred to a dropping funnel 4a a cannula on a second reaction flask. The Grignard reagent was then added dropwise to a solution of sopropypinacolborate (5.30 mL, 26.00 mnmol) in THF (10 mL.) at 0 t. After addition was complete, the reaction mixture was warmed up to ambient temperature and was stirred for 20 hours. The reaction was cooled to -10 CC, and then slowly acetic acid (1.03 mL. 25.50 mmol) was added so that the reaction mixture was at pH 7. Solvent was removed by rotary evaporation then ethyl acetate was added and also removed by rotary evaporation. The crude oil was preabsorbed onto Celite then chromatographed (DCVC) eluting with a gradient of ethyl acetate th heptane (0 - 30% ethyl acetate). Fractions containing the desired material were combined and concentrated to give 2.4-dimethyl-5-(44.5.5-tetramethyl-1 .3,2- dioxaborolan-2-thiaz.ole (9) as a pale yellow oil which solidified (1.65 9, 26%). NMR (400 MHz, DMSO-&)6 2.63 (s. 3H), 2.53 (s. 3H), 1.2 s, 12H). C NMR (50 MHz, DMSO-d10o 170.4.163.21 84.1, 24.9. 19.1.17.6 (one signal not observed). ElMS: rn1z Found: M’ 239.1143, CllHlFNO211B%requires239.i 146. ElMS: rn/z239(M, 66%), 224 (45), 182 7). 139 t,53L 71(100).
References:
WO2016/145478,2016,A1 Location in patent:Paragraph 0099
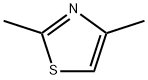
541-58-2
261 suppliers
$6.00/1g

859833-13-9
53 suppliers
$50.00/50mg