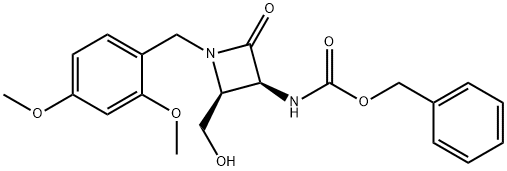
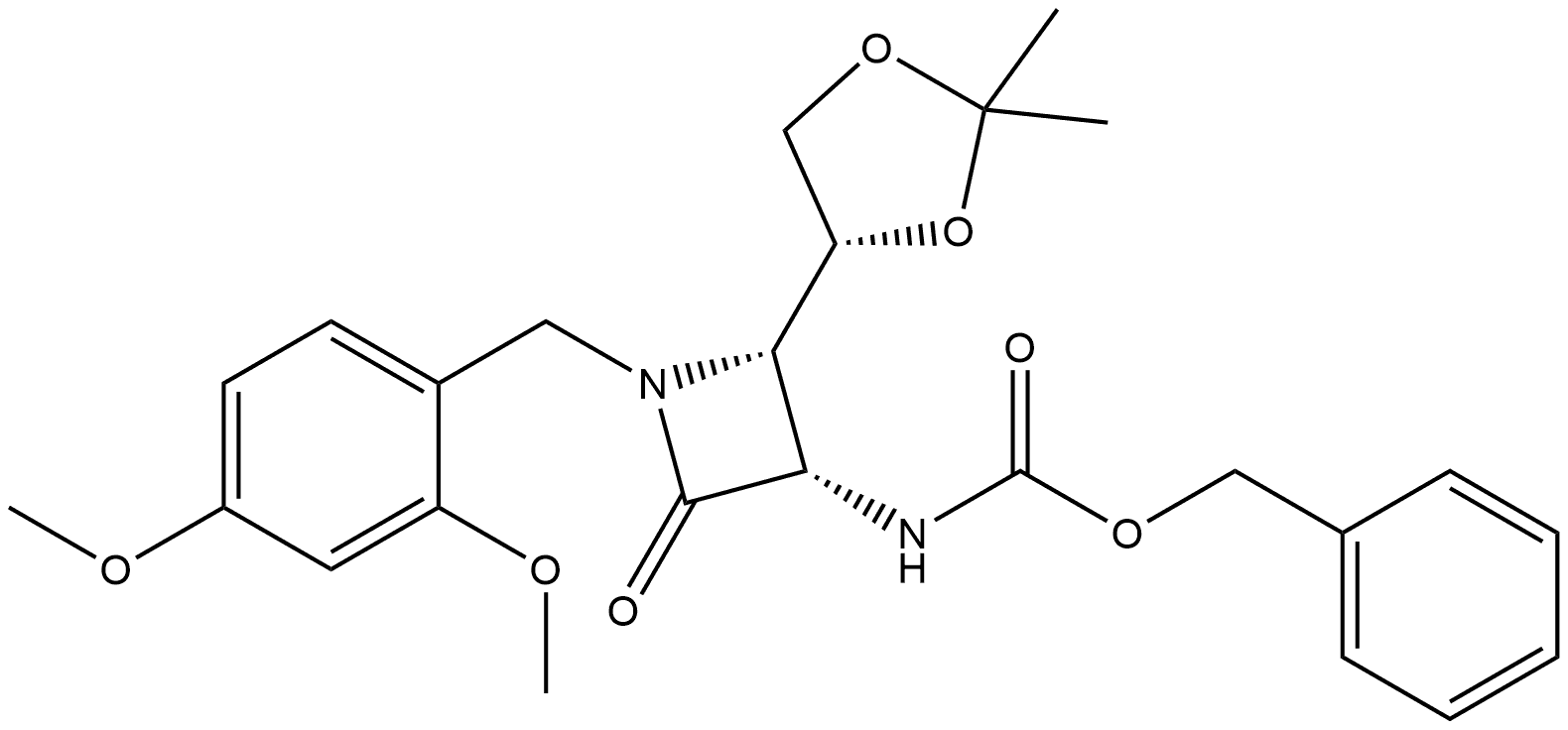
benzyl((2S,3S)-1-(2,4-dimethoxybenzyl)-2-(hydroxymethyl)-4-oxoazetidin-3-yl)carbamate synthesis
- Product Name:benzyl((2S,3S)-1-(2,4-dimethoxybenzyl)-2-(hydroxymethyl)-4-oxoazetidin-3-yl)carbamate
- CAS Number:86334-63-6
- Molecular formula:C21H24N2O6
- Molecular Weight:400.43
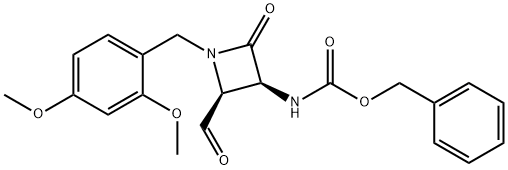
86299-41-4
0 suppliers
inquiry

86334-63-6
6 suppliers
inquiry
Yield:86334-63-6 95%
Reaction Conditions:
with methanol;sodium tetrahydroborate in dichloromethane at 0; for 2 h;
Steps:
7 Step 7:
Benzyl((2S,3S)-1-(2,4-dimethoxybenzyl)-2-(hydroxymethyl)-4-oxoazetidin-3-yl)carbamate
Step 7:
Benzyl((2S,3S)-1-(2,4-dimethoxybenzyl)-2-(hydroxymethyl)-4-oxoazetidin-3-yl)carbamate
To a solution of benzyl((2S,3S)-1-(2,4-dimethoxybenzyl)-2-formyl-4-oxoazetidin-3-yl)carbamate (218 g, 0.546 mol) in a mixture of DCM:MeOH (4:1, 2.25 L) at 0° C. was added sodium borohydride (41.3 g, 1.09 mol), portion-wise.
The resulting mixture was stirred at 0° C. for 2 h, whereupon it was quenched with cold water (1 L) for 30 min and the layers were separated.
The aqueous layer was extracted with DCM (3*200 mL) and the combined organic layers were washed with water, brine, dried over Na2SO4 and concentrated in vacuo to afford the title compound (208 g, 95%) as an off white solid. LCMS: m/z=401.2 (M+1); 1H NMR (300 MHz, CDCl3) δ 7.37-7.29 (m, 5H), 7.21-7.18 (m, 1H), 6.46-6.49 (m, 2H), 5.82 (bd, J=9.6 Hz, 1H), 5.18-5.08 (m, 3H), 4.45 (d, J=14.4 Hz, 1H), 4.28 (d, J=14.4 Hz, 1H), 3.83 (s, 3H), 3.79 (s, 3H), 3.76-3.72 (m, 1H), 3.63-3.52 (m, 2H), 1.87 (dd, J=9.6, 4.0 Hz, 1H).
References:
US2015/266867,2015,A1 Location in patent:Paragraph 0411
20781-20-8
291 suppliers
$8.00/5g

86334-63-6
6 suppliers
inquiry
86334-58-9
0 suppliers
inquiry

86334-63-6
6 suppliers
inquiry
86334-59-0
0 suppliers
inquiry

86334-63-6
6 suppliers
inquiry
![Benzenemethanamine, N-[[(4R)-2,2-dimethyl-1,3-dioxolan-4-yl]methylene]-2,4-dimethoxy-, [N(E)]-](/CAS/20211123/GIF/88852-09-9.gif)
88852-09-9
0 suppliers
inquiry

86334-63-6
6 suppliers
inquiry