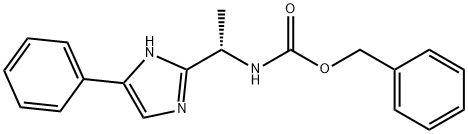
(S)-benzyl 1-(4-phenyl-1H-iMidazol-2-yl)ethylcarbaMate synthesis
- Product Name:(S)-benzyl 1-(4-phenyl-1H-iMidazol-2-yl)ethylcarbaMate
- CAS Number:864825-21-8
- Molecular formula:C19H19N3O2
- Molecular Weight:321.37

864825-19-4
13 suppliers
inquiry

864825-21-8
31 suppliers
inquiry
Yield:864825-21-8 95%
Reaction Conditions:
with ammonium acetate;acetic acid in xylene; for 7 h;Heating / reflux;
Steps:
1.B 2-Amino-3-(4-hydroxy-2,6-dimethyl-phenyl)-N-isopropyl-N-[1-(4-phenyl-1H-imidazol-2-yl)-ethyl]-propionamide
B. [1-(4-Phenyl-1H-imidazol-2-yl)-ethyl]-carbamic acid benzyl ester. To a suspension of [1-(2-oxo-2-phenyl-ethylcarbamoyl)-ethyl]-carbamic acid benzyl ester (2.60 g, 7.64 mmol) in xylene (60 mL) was added NH4OAc (10.3 g, 134 mmol) and HOAc (5 mL). The resulting mixture was heated at reflux for 7 h. After being cooled to room temperature, brine was added and the mixture was separated. The aqueous phase was extracted with EtOAc, and the combined organic phases were dried over Na2SO4 overnight. After filtration and concentration, the residue was purified by column chromatography on silica gel (eluent, EtOAc:hexane-1:1) to give the title compound (2.33 g, 95%). 1H NMR (300 MHz, CDCl3): δ 1.65 (3H, d), 5.06 (1H, m), 5.14 (2H, q), 5.94 (1H, d), 7.32 (10H, m), 7.59 (2H, d). MS(ES+): 322.2 (100%).
References:
US2005/203143,2005,A1 Location in patent:Page/Page column 32
1142-20-7
456 suppliers
$5.00/1g

864825-21-8
31 suppliers
inquiry

5468-37-1
229 suppliers
$17.00/5G

864825-21-8
31 suppliers
inquiry
![L-Alanine, N-[(phenylmethoxy)carbonyl]-, 2-oxo-2-phenylethyl ester](/CAS/20180703/GIF/6530-41-2.gif)
6530-41-2
18 suppliers
inquiry

864825-21-8
31 suppliers
inquiry