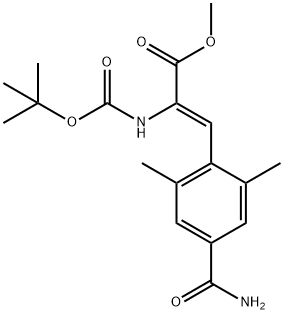
2-Propenoic acid, 3-[4-(aminocarbonyl)-2,6-dimethylphenyl]-2-[[(1,1-dimethylethoxy)carbonyl]amino]-, methyl ester, (2Z)- synthesis
- Product Name:2-Propenoic acid, 3-[4-(aminocarbonyl)-2,6-dimethylphenyl]-2-[[(1,1-dimethylethoxy)carbonyl]amino]-, methyl ester, (2Z)-
- CAS Number:864825-84-3
- Molecular formula:C18H24N2O5
- Molecular Weight:348.39
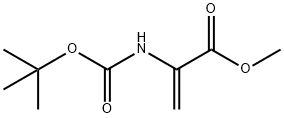
55477-80-0
99 suppliers
$130.00/100mg
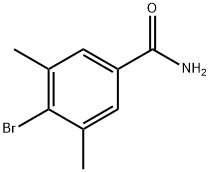
864825-81-0
62 suppliers
$52.00/100mg
![2-Propenoic acid, 3-[4-(aminocarbonyl)-2,6-dimethylphenyl]-2-[[(1,1-dimethylethoxy)carbonyl]amino]-, methyl ester, (2Z)-](/CAS/20180703/GIF/864825-84-3.gif)
864825-84-3
13 suppliers
inquiry
Yield:864825-84-3 57%
Reaction Conditions:
with triethylamine;tris-(o-tolyl)phosphine;tris-(dibenzylideneacetone)dipalladium(0) in N,N-dimethyl-formamide at 110; for 24 h;
Steps:
15.E
E. (Z)-2-tert-Butoxycarbonylamino-3-(4-carbamoyl-2,6-dimethyl-phenyl)acrylic acid methyl ester. A flask charged with Compound 15d (0.46 g, 2.0 mmol), Compound 15f (0.80 g, 4.0 mmol), tri-o-tolylphosphine (0.098 g, 0.32 mmol), DMF (8 mL) was purged with N2 (g) 3 times. After the addition of tris(dibenzylideneacetone)dipalladium (0) (0.074 g, 0.08 mmol) and TEA (0.31 mL, 2.2 mol), the reaction mixture was heated at 110° C. for 24 h. At that time, the reaction was quenched by addition of water, and then extracted with EtOAc. The organic phase was washed with 1 N HCl, saturated aqueous NaHCO3, brine, and dried over MgSO4. The mixture was concentrated to a residue, which was purified by flash column chromatography (eluent: EtOAc:hexane~1:1 to EtOAc only) to give Compound 15g (0.40 g, 57%) as a white solid. 1H NMR (300 MHz, CD3OD): δ 1.36 (9H, s), 2.26 (6H, s), 3.83 (3H, s), 7.10 (1H, s), 7.56 (2H, s); 13C NMR (75 MHz, DMSO-d6): δ 17.6, 25.7, 50.2, 78.7, 124.9, 126.4, 128.3, 131.2, 135.2, 135.5, 152.8, 164.3,169.6; MS (ES+) (relative intensity): 349.1 (38%)(M+1).
References:
US2005/203143,2005,A1 Location in patent:Page/Page column 45