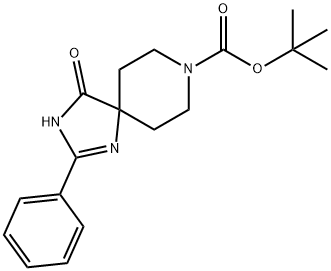
tert-Butyl 4-oxo-2-phenyl-1,3,8-triazaspiro[4.5]dec-1-ene-8-carboxylate synthesis
- Product Name:tert-Butyl 4-oxo-2-phenyl-1,3,8-triazaspiro[4.5]dec-1-ene-8-carboxylate
- CAS Number:865626-62-6
- Molecular formula:C18H23N3O3
- Molecular Weight:329.39
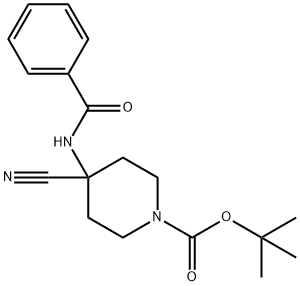
635713-70-1
0 suppliers
inquiry
![tert-Butyl 4-oxo-2-phenyl-1,3,8-triazaspiro[4.5]dec-1-ene-8-carboxylate](/CAS/20140602/GIF/CB52698512.gif)
865626-62-6
8 suppliers
$666.00/1g
Yield:865626-62-6 80%
Reaction Conditions:
with sodium hydroxide;dihydrogen peroxide in ethanol;
Steps:
5 2-Phenyl-1,3,8-triaza-spiro[4.5]dec-1-en-4-one, hydrochloride
To a solution of 4-benzoylamino-4-cyano-piperidine-1-carboxylic acid tertbutyl ester (1.3 g, 4 mmol) in ethanol (10 mL) was added 6M sodium hydroxide (1.5 mL) followed by 30% hydrogen peroxide. The reaction mixture was then refluxed for 3 h. The reaction mixture was then diluted with water (30 mL), and the ethanol removed. The residue was diluted with ethyl acetate (100 mL). The organic phase was washed with brine (30 mL) and dried over sodium sulfate. The desired product, 4-oxo-2-phenyl-1,3,8-triaza-spiro[4.5]dec-1-ene-8-carboxylic acid tert-butyl ester was obtained in 80% yield through crystallization from 30% ethyl acetate in hexane.
References:
US2004/204397,2004,A1
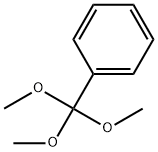
707-07-3
250 suppliers
$5.00/1g

288154-18-7
66 suppliers
$8.00/100mg
![tert-Butyl 4-oxo-2-phenyl-1,3,8-triazaspiro[4.5]dec-1-ene-8-carboxylate](/CAS/20140602/GIF/CB52698512.gif)
865626-62-6
8 suppliers
$666.00/1g