
2-AMINO-5-CHLORO-3-(TRIMETHYLSILYL)ACETYLENYLPYRIDINE synthesis
- Product Name:2-AMINO-5-CHLORO-3-(TRIMETHYLSILYL)ACETYLENYLPYRIDINE
- CAS Number:866318-90-3
- Molecular formula:C10H13ClN2Si
- Molecular Weight:224.76
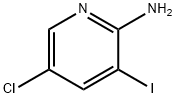
211308-81-5
161 suppliers
$16.00/1g
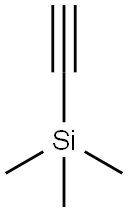
1066-54-2
471 suppliers
$9.00/10g

866318-90-3
28 suppliers
$45.00/10mg
Yield:866318-90-3 100%
Reaction Conditions:
with triethylamine;bis-triphenylphosphine-palladium(II) chloride;copper(l) iodide in tetrahydrofuran at 20; for 2 h;
Steps:
9
A 250mol round bottom flask was charged with 5-chloro-3-iodo-pyridin-2-ylamine (1) (42g, 165mol), THF (100mol), copper iodide (315mg, 1.65mmol) and PdCl2(PPh3)2 (1.15g, 1.65 mmol) under nitrogen. Triethylamine (70ml, 0.5mol), and trimethylsilyl acetylene (30mol, 0.21mol). The reaction mixture was stirred at room temperature for 2h. The reaction mixture was then cooled to 0°C and diethyl ether was added. The suspension was filtered through a celite and thoroughly washed with diethyl ether. The filtrate was concentrated and pre-absorbed onto silica gel and purified by column chromatography using as eluent, pentane/DCM 10% to 100%, to afford an off white solid (36g, 100%). 1H NMR (CDCl3) : 0.3 (9H, s), 5.0-5.1 (2H, brs), 7.6 (1H, s), 7.9 (1H, s). MS (ES+) : 225, 227.
References:
WO2005/95400,2005,A1 Location in patent:Page/Page column 345; 346
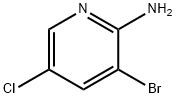
26163-03-1
322 suppliers
$6.00/5g

1066-54-2
471 suppliers
$9.00/10g

866318-90-3
28 suppliers
$45.00/10mg
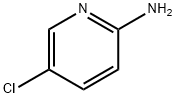
1072-98-6
712 suppliers
$5.00/1g

866318-90-3
28 suppliers
$45.00/10mg