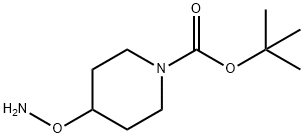
4-Aminooxypiperidine-1-carboxylic acid tert-butyl ester synthesis
- Product Name:4-Aminooxypiperidine-1-carboxylic acid tert-butyl ester
- CAS Number:867034-25-1
- Molecular formula:C10H20N2O3
- Molecular Weight:216.28
![1-Piperidinecarboxylic acid, 4-[(1,3-dihydro-1,3-dioxo-2H-isoindol-2-yl)oxy]-, 1,1-dimethylethyl ester](/CAS/20210305/GIF/143539-99-5.gif)
143539-99-5
0 suppliers
inquiry

867034-25-1
25 suppliers
$45.00/10mg
Yield:867034-25-1 92%
Reaction Conditions:
with methylhydrazine in dichloromethane at 0 - 20; for 16 h;
Steps:
4
Triphenylphosphine (4.17 g, 17.97 mmol) was dissolved in tetrahydrofuran (150 mL) and cooled to 0 °C. N,N-diisopropylazodicarboxylate (3 mL) was added dropwise and 4-hydroxy-piperidin-1-carboxylic acid tert-butyl ester (3 g, 14.9 mmol) was added thereto and the mixture was stirred for 30 minutes. Hydroxyphthalimide (2.46 g, 15.12 mmol) was added and the mixture was stirred at room temperature for 16 hours. The reaction mixture was distilled under reduced pressure to remove the solvent, and purified by column chromatography using 5:5:1 mixture solution of hexane, methylene chloride and ethyl acetate to obtain 4-(1,3-dioxo-1,3-dihydro-isoindol-2-yloxy)-piperidin-1-carboxylic acid tert-butyl ester (3.11 g, 60% yield). The obtained compound (3.11 g, 8.97 mmol) was dissolved in methylene chloride (50 mL) and cooled to 0 °C. Methylhydrazine (0.53 g, 11.67 mmol) was added and the mixture was stirred at room temperature for 16 hours. The reaction mixture was cooled to 0 °C and filtered under reduced pressure with methylene chloride to obtain the title compound (1.8 g, 92% yield).[333] NMR: 1H-NMR(CDCl3) 5.55~5.20(2H, br), 3.81~3.65(3H, m), 3.11~3.01(2H, m), 1.95~1.81(2H, m), 1.58~1.50(2H, m), 1.46(9H, s)
References:
WO2012/111995,2012,A1 Location in patent:Page/Page column 25-26

109384-19-2
475 suppliers
$5.00/1g

867034-25-1
25 suppliers
$45.00/10mg