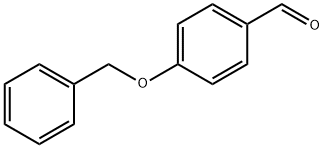
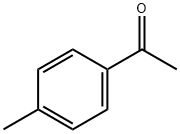
(2E)-3-[4-(benzyloxy)phenyl]-1-(4-methylphenyl)prop-2-en-1-one synthesis
- Product Name:(2E)-3-[4-(benzyloxy)phenyl]-1-(4-methylphenyl)prop-2-en-1-one
- CAS Number:86711-45-7
- Molecular formula:C23H20O2
- Molecular Weight:328.4
Yield:86711-45-7 72%
Reaction Conditions:
with sodium hydroxide in ethanol at 0 - 20;
Steps:
4.2. General procedure for the synthesis of chalcones 1-13
General procedure: To a stirred solution of 4-methyl/4-methoxy acetophenone(0.5 mmol) in EtOH (10 mL), solution of NaOH (60%) (3 mL) was addeddrop-wise by maintaining the temperature at 0 °C. Substituted benzaldehydederivative (0.5 mmol) was added to the above-mentionedmixture and further stirred for 2-3 h. Completion of reaction wasmonitored by TLC analysis. After completion of reaction, it was kept inrefrigerator for overnight and then diluted with ice cold water, theresulting precipitates were filtered and washed with excess of distilledwater. The precipitates were crystallized from methanol [37]. Structuresof compounds 1-13 were deduced by EI-MS and 1H NMR spectroscopictechniques. CHN analysis was also performed
References:
Tajudeen Bale, Adebayo;Mohammed Khan, Khalid;Salar, Uzma;Chigurupati, Sridevi;Fasina, Tolulope;Ali, Farman;Kanwal;Wadood, Abdul;Taha, Muhammad;Sekhar Nanda, Sitanshu;Ghufran, Mehreen;Perveen, Shahnaz [Bioorganic Chemistry,2018,vol. 79,p. 179 - 189]