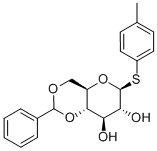
4-Methylphenyl 4,6-O-benzylidene-1-thio-b-D-glucopyranoside synthesis
- Product Name:4-Methylphenyl 4,6-O-benzylidene-1-thio-b-D-glucopyranoside
- CAS Number:868241-49-0
- Molecular formula:C20H22O5S
- Molecular Weight:374.45
Yield:868241-49-0 162 g
Reaction Conditions:
with camphor-10-sulfonic acid in acetonitrile at 55;
Steps:
2.4 Formation of Monosaccharide D-4
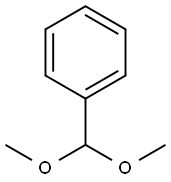
D-3 (235.5 g) in step 3 was dissolved in acetonitrile (ACN) (1398 ml) at 55° C. DL-10-camphorsulfonic acid (CSA) (38.2 g), Benzaldehyde dimthylactal (137.7 g) were added to the solution and stirred for 3-4 hours until completion checked by TLC (MeOH/DCM=1/3; EA/hexane=1/1). Trithylamine (17 g) was added to quench the reaction at 50° C. The solution was neutralized with saturated sodium bicarbonate aqueous solution, (pH 23→pH 78). Hexanes (1300 ml) was added for extracting Benzaldehyde dimthylactal off the solution at 50° C. Pure white powder (162 g)was obtained by recrystallization.
References:
US2017/15695,2017,A1 Location in patent:Paragraph 0128; 0129