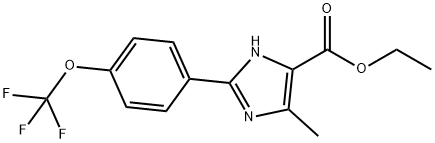
5-METHYL-2-(4-TRIFLUOROMETHOXYPHENYL)-3H-IMIDAZOLE-4-CARBOXYLIC ACID ETHYL ESTER synthesis
- Product Name:5-METHYL-2-(4-TRIFLUOROMETHOXYPHENYL)-3H-IMIDAZOLE-4-CARBOXYLIC ACID ETHYL ESTER
- CAS Number:868851-35-8
- Molecular formula:C14H13F3N2O3
- Molecular Weight:314.26

93919-56-3
155 suppliers
$10.00/1g

5408-04-8
65 suppliers
$32.00/500mg

868851-35-8
17 suppliers
inquiry
Yield:-
Reaction Conditions:
in acetonitrile; for 17 h;Heating / reflux;
Steps:
1 Preparation of 5-methyl-2-(4-trifluoromethoxy-phenyl)-1H-imidazole-4-carboxylic acid ethyl ester
To a solution of 7.9 g of ethyl 2-oximinoacetoacetate in acetonitrile (100 ml) was added 10.5 g of 4-(trifluoromethoxy)benzylamine (as R4-CH2-NH2). The reaction mixture was then refluxed for 17 hours under argon atmosphere. After such time the reaction mixture was cooled down to 0° C., the solid was filtered off, washed with acetonitrile, and dried in vacuo to yield 11.2 g of a light yellow powder, MS (ISP) 315 (M+H)+.
References:
US2005/250769,2005,A1 Location in patent:Page/Page column 14