
4-(5-chloro-2-(isopropylaMino)pyridin-4-yl)-1H-pyrrole-2-carboxylic acid synthesis
- Product Name:4-(5-chloro-2-(isopropylaMino)pyridin-4-yl)-1H-pyrrole-2-carboxylic acid
- CAS Number:869886-90-8
- Molecular formula:C13H14ClN3O2
- Molecular Weight:279.72
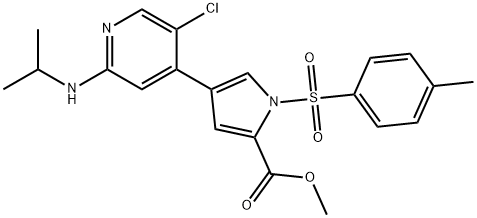
869886-88-4
1 suppliers
inquiry

869886-90-8
12 suppliers
inquiry
Yield:869886-90-8 74%
Reaction Conditions:
with lithium hydroxyde monohydrate;diethylamine in tetrahydrofuran;water monomer at 15 - 70; for 30 h;Inert atmosphere;Reflux;Large scale;
Steps:
2.3
In Step 3, a clean and dry 300 L glass-lined reactor was evacuated to -0.08 MPa, and then filled with nitrogen to normal pressure three times. THE (62.58 kg) was charged into the 300 L glass-lined reactor at 15-30°C. Then the stirrer was started. ASYM-1 12393 (12.00 kg, 11.70 kg after corrected) was added into the mixture. The mixture was stirred until the solid dissolved completely. Maintaining the temperature at 15-30°C, a lithium hydroxide solution which wasprepared with lithium hydroxide monohydrate (5.50 kg) in purified water (70.28 kg) was added into the mixture. Then diethylamine (3.86 kg) was added. The mixture was heated to 60-70°C for refluxing. The mixture reacted at 60-70°C. After 30 h, the reaction was sampled and analyzed by HPLC every 4-6 h until the content of intermediate at relative retention time (RRT)=1 .39-1.44 was <1 % and the content of ASYM-1 12393 was <1 %. HPLC conditions for this analysis are set forth in Table 1.
References:
WO2016/123574,2016,A1 Location in patent:Paragraph 0228; 0231
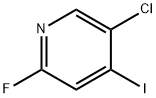
659731-48-3
141 suppliers
$12.00/250mg

869886-90-8
12 suppliers
inquiry
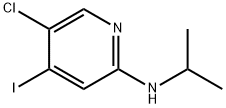
869886-87-3
12 suppliers
inquiry

869886-90-8
12 suppliers
inquiry
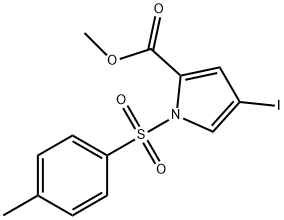
869886-85-1
20 suppliers
inquiry

869886-90-8
12 suppliers
inquiry

16110-09-1
453 suppliers
$5.00/1g

869886-90-8
12 suppliers
inquiry