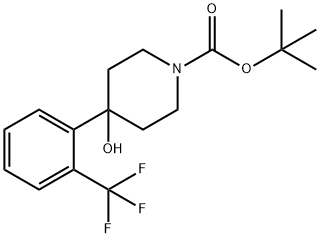
1-BOC-4-[2-(TRIFLUOROMETHYL)PHENYL]-4-HYDROXYPIPERIDINE synthesis
- Product Name:1-BOC-4-[2-(TRIFLUOROMETHYL)PHENYL]-4-HYDROXYPIPERIDINE
- CAS Number:871112-34-4
- Molecular formula:C17H22F3NO3
- Molecular Weight:345.36
Yield:871112-34-4 85%
Reaction Conditions:
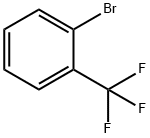
with N-ethyl-N,N-diisopropylamine in dichloromethane at 20; for 4 h;
Steps:
22.A
Step A: To a solution of 4- (2- ( rifluoromethyl ) phenyl) iperidin-4 -ol (1.00 g, 4.08 mmol) in CH2C12 (25 mL) and i-Pr2NEt (1.0 mL, 5.74 mmol) was added di-tert-butyl dicarbonate (1.07 g 4.90 mmol) and the reaction stirred at ambient temperature for 4 hours. The reaction was diluted with aqueous saturated NHaCl and extracted with (2 x 25 mL) . The combined organic extracts were washed with H20, dried over MgS04, filtered, and concentrated under reduced pressure to provide tert-butyl 4-hydroxy-4- (2- (trifluoromethyl) phenyl ) piperidine-1-carboxylate as an off-white solid (1.20 g, 85%): NMR (300 MHz, CDCla) 5 7.79 (d, J = 7.8 Hz, 1H) , 7.53-7.51 (m, 2H) , 7.40-7.34 (m, 1H) , 4.09-4.02 (m, 2H) , 3.31-3.20 (m, 2H) , 2.16-2.05 (m, 2H), 1.96-1.77 (m, 3H) , 1.48 (s, 9H) .
References:
WO2014/152013,2014,A1 Location in patent:Page/Page column 131

79099-07-3
543 suppliers
$5.00/5g
![1-BOC-4-[2-(TRIFLUOROMETHYL)PHENYL]-4-HYDROXYPIPERIDINE](/CAS/GIF/871112-34-4.gif)
871112-34-4
6 suppliers
inquiry
![4-[2-(TRIFLUOROMETHYL)PHENYL]-4-PIPERIDINOL](/CAS/GIF/69908-12-9.gif)

![Lithium, [2-(trifluoromethyl)phenyl]-](/CAS/20211123/GIF/49571-31-5.gif)