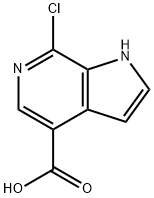
1H-Pyrrolo[2,3-c]pyridine-4-carboxylic acid, 7-chloro- synthesis
- Product Name:1H-Pyrrolo[2,3-c]pyridine-4-carboxylic acid, 7-chloro-
- CAS Number:871819-31-7
- Molecular formula:C8H5ClN2O2
- Molecular Weight:196.59

124-38-9
122 suppliers
$214.00/14L
![1H-Pyrrolo[2,3-c]pyridine-4-carboxylic acid, 7-chloro-](/CAS/GIF/871819-31-7.gif)
871819-31-7
6 suppliers
inquiry
Yield:-
Reaction Conditions:
Stage #1: 4-bromo-1-(tert-butyl-dimethyl-silanyl)-7-chloro-1H-pyrrolo[2,3-c]pyridinewith tert.-butyl lithium in tetrahydrofuran;pentane at -78; for 0.25 h;
Stage #2: carbon dioxide in tetrahydrofuran;pentane;
Stage #3: with hydrogenchloride in water;
Steps:
19.d
To a solution of 4-bromo-l-(tert-butyl-dimethyl-silanyl)-7-chloro-lH-pyrrolo[2,3-clpyridine (500mg) in dry tetrahydrofuran (25ml) at -78 °C under an atmosphere of nitrogen was added tert-butyllithium (1.7M in pentane, 1.88ml). After addition, the reaction mixture was stirred for 15 minutes at -78 °C then poured onto crushed pellets of carbon dioxide. The mixture was allowed to warm to room temperature and then evaporated. The residue was dissolved in water and the aqueous washed twice with diethyl ether. The aqueous was then acidified with 2M hydrochloric acid and extracted twice with ethyl acetate. The organic layer was dried (MgS04) and evaporated to give the title compound as a yellow solid (120mg). NMR (d6-DMSO) No. 0.62 (6H, s), 0.87 (9H, s), 6.64 (1H, d), 7.45 (lH, d), 8.03 (1H, s). LC/MS t = 1.8min, [MH+] 197 consistent with molecular formula G8H535ClN2O2
References:
WO2005/121140,2005,A1 Location in patent:Page/Page column 46-47