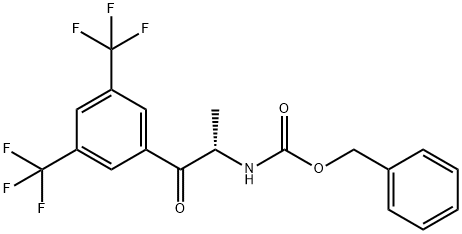
CarbaMic acid, N-[(1S)-2-[3,5-bis(trifluoroMethyl)phenyl]-1-Methyl-2-oxoethyl]-, phenylMethyl ester synthesis
- Product Name:CarbaMic acid, N-[(1S)-2-[3,5-bis(trifluoroMethyl)phenyl]-1-Methyl-2-oxoethyl]-, phenylMethyl ester
- CAS Number:871917-79-2
- Molecular formula:C19H15F6NO3
- Molecular Weight:419.32
Yield:99.9 % ee
Reaction Conditions:
Stage #1: 3,6-bis(trifluoromethyl)bromobenzene;(S)-benzyl (1-(N,O-dimethylhydroxylamine)-1-oxopropan-2-yl)carbamatewith isopropylmagnesium chloride in tetrahydrofuran at -10 - 20;
Stage #2: with hydrogenchloride in tetrahydrofuran;water at 0 - 5; for 2 h;
Steps:
2
The Weinreb amide from Step 1 (6kg, 22.5 mol) and 3,5-bis(trifluoromethyl)bromobenzene (4.85L, 28.1mol) are dissolved in anhydrous THF (24L). The solution is purged with nitrogen to remove distilledoxygen. The solution is cooled to -10°C and iso-PrMgCl in THF (56.4 mol) is slowly added (2 hours) tothe reaction via addition funnel, maintaining a reaction temperature < - 5 °C. The solution is allowed to warm to 20°C and aged overnight at 20° C. The reaction is then cooled to -10°C under nitrogen and isquenched slowly over 2 hours into 5N HC1 (14L) that is maintained at 0-5°C. MTBE (60L) is added andthe biphasic mixture is agitated for 5 min. After warming to 20°-25°C, it is allowed to settle for 30 min,and then the layers are separated. The organic layer is washed with water twice (12L).The organic layer is vacuum transferred through a 1-micron in-line PTFE filter into a distillation flaskand is then concentrated to ~12L under vacuum (internal temperature <40°C). Heptane is added and thedistillation is continued under vacuum at 40°-55°C until the final volume is 40L. The solution is cooledto 35°-37"C , seeded (-0.5%, 30gms) and then aged for 30min to allow for a full seed bed to grow. Theslurry is cooled to 10°C over 2-3 hrs. The slurry is then filtered, washed with 5°C heptane (18L), andallowed to dry fully on the filter pot using a vacuum/nitrogen sweep overnight. The dried solid isobtained with >99.9ee%. The amide can be recrystallized from straight heptane if the optical purity isnot sufficient.
References:
WO2006/14357,2006,A1 Location in patent:Page/Page column 50-51

328-70-1
438 suppliers
$10.00/25g

37661-60-2
26 suppliers
$125.00/250mg
![CarbaMic acid, N-[(1S)-2-[3,5-bis(trifluoroMethyl)phenyl]-1-Methyl-2-oxoethyl]-, phenylMethyl ester](/CAS/20150408/GIF/871917-79-2.gif)
871917-79-2
2 suppliers
inquiry

112981-69-8
35 suppliers
$129.00/50ml

114744-83-1
25 suppliers
inquiry
![CarbaMic acid, N-[(1S)-2-[3,5-bis(trifluoroMethyl)phenyl]-1-Methyl-2-oxoethyl]-, phenylMethyl ester](/CAS/20150408/GIF/871917-79-2.gif)
871917-79-2
2 suppliers
inquiry
1142-20-7
464 suppliers
$5.00/1g
![CarbaMic acid, N-[(1S)-2-[3,5-bis(trifluoroMethyl)phenyl]-1-Methyl-2-oxoethyl]-, phenylMethyl ester](/CAS/20150408/GIF/871917-79-2.gif)
871917-79-2
2 suppliers
inquiry