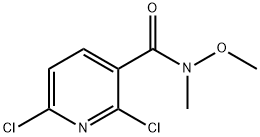
2,6-Dichloro-N-methoxy-N-methylpyridine-3-carboxamide synthesis
- Product Name:2,6-Dichloro-N-methoxy-N-methylpyridine-3-carboxamide
- CAS Number:873936-98-2
- Molecular formula:C8H8Cl2N2O2
- Molecular Weight:235.07
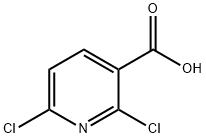
38496-18-3
364 suppliers
$6.00/1g

6638-79-5
559 suppliers
$6.00/25g

873936-98-2
17 suppliers
inquiry
Yield:873936-98-2 81%
Reaction Conditions:
Stage #1: 2,6-dichloropyridine-3-carboxylic acid;N,O-dimethylhydroxylamine*hydrochloridewith benzotriazol-1-ol;1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride in dichloromethane at 0; for 0.25 h;
Stage #2: with triethylamine in dichloromethane at 0 - 20; for 16 h;
Steps:
12a 26-Dichloro-N-methoxy-N-methylnicotinamide
O,N-Dimethyl-hydroxylamine hydrochloride (19 g, 0.195 mol), EDCxHCI (27.4 g, 0.143 mol) and HOBT (19.32 g, 0.143 mol) were added at 0°C to a stirred suspension of 2,6-dichloro-nicotinic acid (25 g, 0.13mol) in DCM (450 ml). The reaction mixture was stirred for 15 mm, TEA (72 ml, 0.52 mol) was added drop wise at 0°C, and stirring was continued at RT for 16 h. The reaction mixture was washed successively with water, saturated NaHCO3 solution, saturated NH4CI solution and brine, dried over Na2SO4 and concentrated. The remnant was purified by column chromatography [100-200 mesh silica gel, hexane/EtOAc = 9:1]. White solid. Yield: 25 g (81%). 1H NMR (400 MHz, DMSO-d6, ? ppm): 8.11-8.09(d, 1H), 7.70-7.68 (d, 1H), 3.65-3.47 (m, 3H), 3.30-3.12 (m, 3H).
References:
WO2018/234353,2018,A1 Location in patent:Page/Page column 43

6638-79-5
559 suppliers
$6.00/25g
58584-83-1
32 suppliers
$45.95/1gm:

873936-98-2
17 suppliers
inquiry

38496-18-3
364 suppliers
$6.00/1g

873936-98-2
17 suppliers
inquiry