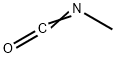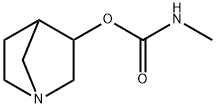
1-Azabicyclo[2.2.1]heptan-3-ol, 3-(N-methylcarbamate) synthesis
- Product Name:1-Azabicyclo[2.2.1]heptan-3-ol, 3-(N-methylcarbamate)
- CAS Number:874148-31-9
- Molecular formula:C8H14N2O2
- Molecular Weight:170.21
Yield:874148-31-9 430 mg (63%)
Reaction Conditions:
with pyridine in tetrahydrofuran;methanol;ethanol;ethyl acetate;
Steps:
1 (exo)-Methylcarbamic acid 1-aza-bicyclo[2.2.1]hept-3-yl ester
EXAMPLE 1 (exo)-Methylcarbamic acid 1-aza-bicyclo[2.2.1]hept-3-yl ester A solution of 452 mg (4 mmole) of (exo-)-1-azabicyclo[2.2.1]heptan-3-ol in 15.0 mL dry THF and 3.5 mL dry pyridine was treated with methyl isocyanate (800 mg, 14 mmole, 0.8 mL) and stirred at 50°-60° C. under nitrogen 24 hours and then at room temperature for 2 days. Removal of solvent at reduced pressure and flash chromatography through alumina using methanol (2-10%) in ethyl acetate yielded 430 mg (63%) of title compound as a waxy white solid, mp: 85-95 C. A second purification on alumina using ethanol (1-6%) in ethyl acetate gave 162 mg of a waxy solid, mp: 92°-96° C. MS (EI, m/z): 170 (M+) plus 71 (CH3NCO, M+). Elemental analysis for C8 H14 N2 O2. 0.33 CH3 NCO Calc'd: C, 55.02; H, 8.00; N, 17.26 Found: C, 55.00; H, 8.19; N, 17.21
References:
US5468875,1995,A
![1-azabicyclo[2.2.1]heptan-3-ol](/CAS/20200119/GIF/138741-10-3.gif)
138741-10-3
5 suppliers
inquiry
![1-Azabicyclo[2.2.1]heptan-3-ol, 3-(N-methylcarbamate)](/CAS/20210111/GIF/874148-31-9.gif)
874148-31-9
1 suppliers
inquiry