
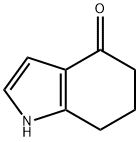
4,5,6,7-Tetrahydro-4-oxo-1H-indole-1-carboxylic acid 1,1-dimethylethyl ester synthesis
- Product Name:4,5,6,7-Tetrahydro-4-oxo-1H-indole-1-carboxylic acid 1,1-dimethylethyl ester
- CAS Number:877170-76-8
- Molecular formula:C13H17NO3
- Molecular Weight:235.28
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry
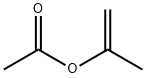
108-22-5
244 suppliers
$19.00/25mL

877170-76-8
21 suppliers
inquiry
Yield: 26%
Reaction Conditions:
with toluene-4-sulfonic acid for 5 h;Heating / reflux;
Steps:
3
Tert-butyl 4,5,6,7-tetrahydro-4-oxoindole-1-carboxylate 4,5,6,7-tetrahydro-4-oxoindole-1-carboxylate (1) was prepared using a modified literature procedure (Maekawa, which is hereby incorporated by reference). To a dry 250 mL round bottom flask charged with a magnetic stir bar was added tert-butyl 4,5,6,7-tetrahydro-4-oxoindole-1-carboxylate (4.619 g, 19.6 mmole), 150 mL isopropenyl acetate and p-toluenesulfonic acid monohydrate (67 mg, 0.35 mmole). The solution was refluxed for 5 hours while the solvent was slowly distilled off at the rate of 20 mL/hour. After cooling the solution to ambient temperature, 100 mL of a 1:1 solution of diethyl ether and saturated aqueous NaHCO3 was added. The layers were separated and the aqueous layer extracted with 30 mL diethyl ether. The combined ether extracts were washed with saturated aqueous NaHCO3 (2×30 mL), brine (30 mL), and dried over anhydrous MgSO4. The solution was then filtered through a plug of silica gel and concentrated in vacuo. The residue was purified via flash chromatography (silica gel, 5:1 pentane:diethyl ether) to give the product as a white solid (1.40 g, 26% yield), mp 79-80° C.; Rf 0.26 (5:1 pentane:diethyl ether); FTIR (neat): 2978, 1736 cm-1; 1H NMR (500 MHz, CDCl3) δ 7.09 (d, J=3.5 Hz, 1H), 5.95 (d, J=3.5 Hz, 1H), 5.24 (t, J=4.5 Hz, 1H), 3.10 (t, J=9.5 Hz, 2H), 2.56 (dt, J=9.5, 4.5 Hz, 2H), 2.23 (s, 3H), 1.58 (s, 9H); 13C NMR (75 MHz, CDCl3) δ 169.1 (C), 149.0 (C), 143.3 (C), 130.8 (C), 119.9 (CH), 118.9 (C), 106.8 (CH), 105.4 (CH), 83.8 (C), 27.9 (CH3), 23.1 (CH2), 21.7 (CH2), 20.7 (CH3); LCMS (ESI) m/z (relative intensity): 250.0 (100), 236.0 (90); HRMS (EI) Calcd for C15H19NO4 277.1309, Found 277.1309.
References:
Davies, Huw M. L.;Manning, James US2007/4787, 2007, A1 Location in patent:Page/Page column 23
13754-86-4
148 suppliers
$20.00/5g

24424-99-5
821 suppliers
$13.50/25G

877170-76-8
21 suppliers
inquiry

504-02-9
555 suppliers
$5.00/10g

877170-76-8
21 suppliers
inquiry
![2-Cyclohexen-1-one, 3-[(2-hydroxyethyl)amino]-](/CAS/20210305/GIF/16179-66-1.gif)
16179-66-1
0 suppliers
inquiry

877170-76-8
21 suppliers
inquiry