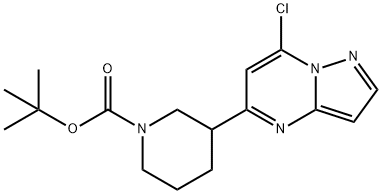
tert-butyl 3-(7-chloropyrazolo[1,5-a]pyrimidin-5-yl)piperidine-1-carboxylate synthesis
- Product Name:tert-butyl 3-(7-chloropyrazolo[1,5-a]pyrimidin-5-yl)piperidine-1-carboxylate
- CAS Number:877173-81-4
- Molecular formula:C16H21ClN4O2
- Molecular Weight:336.82
![tert-butyl 3-(7-hydroxypyrazolo[1,5-a]pyrimidin-5-yl)piperidine-1-carboxylate](/CAS/20180702/GIF/891494-66-9.gif)
891494-66-9
10 suppliers
inquiry
![tert-butyl 3-(7-chloropyrazolo[1,5-a]pyrimidin-5-yl)piperidine-1-carboxylate](/CAS/20180702/GIF/877173-81-4.gif)
877173-81-4
9 suppliers
inquiry
Yield:877173-81-4 71%
Reaction Conditions:
with trichlorophosphate in N,N-dimethyl-aniline at 25; for 96 h;Product distribution / selectivity;
Steps:
19; X-30-C
A mixture of the product from Preparative Example 2 (12.50 g, 39.3 mmol), N,N-dimethylaniline (15.5 mL), and POCl3 (125 mL) was stirred at 25° C. for 4 days. Excess of POCl3 was evaporated and the residue was poured into saturated aqueous NaHCO3 (600 mL). The mixture was extracted with CH2Cl2 (3×200 mL), the combined extracts were dried over Na2SO4, filtered, and the solvent was evaporated. The residue was purified by column chromatography on silica gel with 8:1 CH2Cl2/EtOAc as eluent. Pale yellow wax (9.41 g, 71%) was obtained. LC-MS: 337 [M+].; PREPARATIVE EXAMPLE X-30-C A mixture of the product from Preparative Example X-20-C (12.50 g, 39.3 mmol), N,N-dimethylaniline (15.5 mL), and POCl3 (125 mL) was stirred at 25° C. for 4 days. Excess of POCl3 was evaporated and the residue was poured into saturated aqueous NaHCO3 (600 mL). The mixture was extracted with CH2Cl2 (3×200 mL), the combined extracts were dried over Na2SO4, filtered, and the solvent was evaporated. The residue was purified by column chromatography on silica gel with 8:1 CH2Cl2/EtOAc as eluent. Pale yellow wax (9.41 g, 71%) was obtained. LC-MS: 337 [M+].
References:
US2007/82900,2007,A1 Location in patent:Page/Page column 98; 113
891494-65-8
35 suppliers
$56.00/250mg
![tert-butyl 3-(7-chloropyrazolo[1,5-a]pyrimidin-5-yl)piperidine-1-carboxylate](/CAS/20180702/GIF/877173-81-4.gif)
877173-81-4
9 suppliers
inquiry