
(S)-tert-Butyl 1-(tetrahydro-2H-pyran-4-yl)pyrrolidin-3-ylcarbamate synthesis
- Product Name:(S)-tert-Butyl 1-(tetrahydro-2H-pyran-4-yl)pyrrolidin-3-ylcarbamate
- CAS Number:877661-68-2
- Molecular formula:C14H26N2O3
- Molecular Weight:270.37
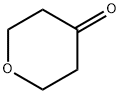
29943-42-8
426 suppliers
$5.00/1g
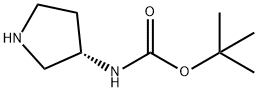
122536-76-9
289 suppliers
$7.00/1g

877661-68-2
8 suppliers
$370.00/1g
Yield:877661-68-2 98%
Reaction Conditions:
Stage #1: Tetrahydro-4H-pyran-4-one;3(S)-(tert-butoxycarbonylamino)pyrrolidinewith acetic acid in dichloromethane at 25; for 1 h;
Stage #2: with sodium tris(acetoxy)borohydride in dichloromethane at 25; for 2 h;
Stage #3: with sodium hydrogencarbonate in dichloromethane;water at 25; for 4 h;
Steps:
4.a
4 g (21.47 mmol) (S)-3-Boc-amino-pyrrolidine and 2.37 g (23.62 mmol) tetrahydro-pyran-4-one are dissolved in 50 ml dichloromethane and combined with 0.43 ml (7.51 mmol) glacial acetic acid. The mixture is stirred for 1 h at 25° C. and then 0.8 ml (13.96 mmol) glacial acetic acid and batchwise 9.1 g (42.94 mmol) sodium trisacetoxyborohydride are added. After 2 h at 25° C. the mixture is combined with 50 ml saturated sodium hydrogen carbonate solution. This mixture is stirred for 4 h at 25° C. Then the phases are separated. The aqueous phase is again extracted with 50 ml dichloromethane. The combined organic phases are dried and the solvent is eliminated in vacuo. Yield: 5.72 g (21.19 mmol, 98%)
References:
US2006/47118,2006,A1 Location in patent:Page/Page column 10