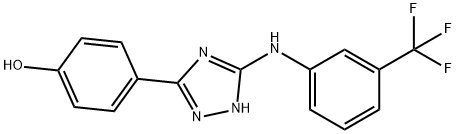
4-(5-(3-(TRIFLUOROMETHYL)PHENYLAMINO)-4H-1,2,4-TRIAZOL-3-YL)PHENOL synthesis
- Product Name:4-(5-(3-(TRIFLUOROMETHYL)PHENYLAMINO)-4H-1,2,4-TRIAZOL-3-YL)PHENOL
- CAS Number:877874-79-8
- Molecular formula:C15H11F3N4O
- Molecular Weight:320.27
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry
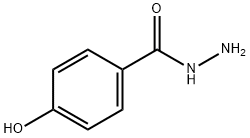
5351-23-5
210 suppliers
$23.00/5 g

877874-79-8
10 suppliers
$665.67/1g
Yield: 58.84%
Reaction Conditions:
with 2,6-dimethylpyridine in acetonitrile at 20 - 80;Inert atmosphere;
Steps:
1.6 Step-6: Preparation of the scaffold-Method 2
To a suspension of methyl S-Methyl-3-(trifluoromethyl) phenylisothiourea hydroiodide (6 g, 16.57 mmole) in Acetonitrile (60 mL) was added 2,6-lutidine(3.53 g, 33 mmol) under N2↑ atmosphere at room temperature followed by 4-hydroxy benzohydrazide(3.02 g, 19.8 mmol) and stirred at room temperature for 15 min. After complete addition of hydrazide reaction mixture was stirred at 80° C. for 18-20 hrs. When TLC (mobile phase-10% methanol in chloroform, Rf. S.M.-0. 0.20, product-0.5) showed absence of starting material and formation of product, the mixture was cooled to room temperature and acetonitrile was removed under vacuum. The residue was taken in ethyl acetate and the organic layer was washed with water (2×), followed by 10% citric acid solution (2×) and finally with brine. Organic layer was separated and dried over Na2SO4 (solid) and concentrated to give crude product. The crude product was purified by column chromatography to afford desired product (3.1 g). (0355) Yield: 58.84% (0356) HPLC Purity: 96%. (0357) LCMS: MH+321 (Mol. Wt 320)
References:
AMITECH THERAPEUTIC SOLUTIONS, INC.;Arnold, Lee Daniel;Murphy, Eric A. US9212151, 2015, B2 Location in patent:Page/Page column 87-88

98-16-8
382 suppliers
$9.00/5g

877874-79-8
10 suppliers
$665.67/1g
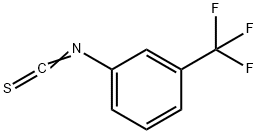
1840-19-3
94 suppliers
$17.00/1g

877874-79-8
10 suppliers
$665.67/1g

1736-70-5
99 suppliers
$10.00/1g

877874-79-8
10 suppliers
$665.67/1g