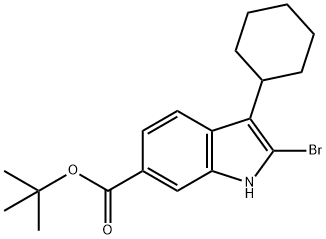
tert-butyl 2-bromo-3-cyclohexyl-1H-indole-6-carboxylate synthesis
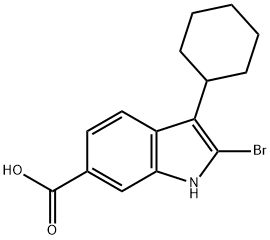
- Product Name:tert-butyl 2-bromo-3-cyclohexyl-1H-indole-6-carboxylate
- CAS Number:879498-90-5
- Molecular formula:C19H24BrNO2
- Molecular Weight:378.3
Yield:879498-90-5 85%
Reaction Conditions:
silver carbonate in tetrahydrofuran;dichloromethane at 0 - 20;Molecular sieve 4A;
Steps:
Intermediate 15
1H-Indole-6-carboxylic acid, 2-bromo-3-cyclohexyl-, 1,1-dimethylethyl ester. To a mechanically stirred solution of 2-bromo-3-cyclohexyl-1H-indole-6-carboxylic acid (80 g, 0.24 m) in dry methylene dichloride(1.2 L) and THF (100 mL) were added activated molecular sieves (4A, 80 g) and silver carbonate (275 g, 0.99 m). The reaction mixture was cooled to 0 C. and t-Butyl bromide (142 g, 1.04 m) was added drop wise. The mixture was stirred overnight at rt and monitored by TLC (Hexane-Ethyl acetate 80:20, Rf (Product)=0.7). If any bromo acid was left unconverted a further 10% of silver carbonate was added and stirring was continued for an addition 2-4 h. On completion, the reaction mixture was filtered through a thin bed of celite. The filtrand was washed with methylene dichloride (500 mL). The combined filtrates were concentrated in-vacuo, and the crude product thus obtained was purified by silica gel chromatography:(230-400 mesh, eluted with a gradient of ethyl acetate in pet ether 0-2%). Homogeneous fractions were combined and evaporated under reduced pressure to give 80 g (85%) of the title compound. HPLC: 90.1% (RT=6.56 min), Column: C18 BDS, (50 4.6 mm), Mobile Phase: Gradient of 0.1% TFA in water: ACN (30?100?30), Flow rate 0.8 mL/min. LCMS: 99.8% (RT=4.44 min), Column: Geneis, C18 50 4.6 mm Mobile Phase: Gradient of 0.1% Formic acid in water : ACN (70?95?70), Flow rate: 0.8 mL/min; M-1=376.5; 1H NMR CDCl3) (400 MHz) ? 1.37-1.40 (m, 3H, cyc.Hexyl), 1.62 (s, 9H, t-Bu), 1.80-1.94 (two sets of m, 3H, & 4H respectively, cyc.Hexyl part), 2.81 (m, 1H, CH of cyc.Hexyl-benzylic), 7.70-7.75 (m, 2H, Indole-H4&5), 8.04 (s, 1H, Indole-H7), 8.52 (s, 1H, Indole-NH).
References:
US2007/275930,2007,A1 Location in patent:Page/Page column 22-23
879498-89-2
0 suppliers
inquiry

879498-90-5
17 suppliers
inquiry
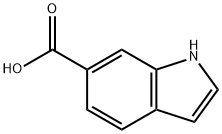
1670-82-2
352 suppliers
$10.00/1g

879498-90-5
17 suppliers
inquiry

108-94-1
546 suppliers
$12.00/50g

879498-90-5
17 suppliers
inquiry