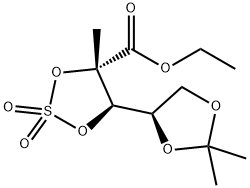
(4S,5R)-ethyl 5-((R)-2,2-diMethyl-1,3-dioxolan-4-yl)-4-Methyl-1,3,2-dioxathiolane-4-carboxylate 2,2-dioxide synthesis
- Product Name:(4S,5R)-ethyl 5-((R)-2,2-diMethyl-1,3-dioxolan-4-yl)-4-Methyl-1,3,2-dioxathiolane-4-carboxylate 2,2-dioxide
- CAS Number:879551-01-6
- Molecular formula:C11H18O8S
- Molecular Weight:310.32
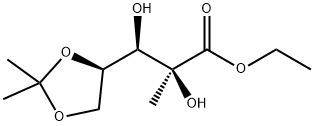
93635-76-8
142 suppliers
$10.00/5g

879551-01-6
12 suppliers
inquiry
Yield:879551-01-6 93%
Reaction Conditions:
Stage #1: ethyl (2S,3R)-3-((4R)-2,2-dimethyl-1,3-dioxolan-4-yl)-2,3-dihydroxy-2-methylpropanoatewith dichloro sulfoxide in Carbon tetrachloride;acetonitrile at 0; for 0.166667 h;
Stage #2: with sodium (meta)periodate;triethylamine in Carbon tetrachloride;lithium hydroxide monohydrate;acetonitrile at 20; for 0.333333 h;Reagent/catalyst;
Steps:
3 Example 3:
Oily compound I(2S,3R)-3-[(4R)-2,2-dimethyl-1,3dioxolane-2,3-diol-2-methyl-propionic acid ethyl ester 50g dissolved In 450ml of carbon tetrachloride andIn a mixed solvent of 450 ml of acetonitrile,Add Au25 (SC2H4Ph)18/TiO20.7g (nano clusters accounted for 10% by weight of the porous carrier),Add SOCl226g at 0 °C,The reaction was carried out at 0 ° C for 10 min, and then 300 mL of a 0.2 mol/L triethylamine aqueous solution was added, and the temperature was naturally raised to room temperature.Add 6.5 g of sodium periodate (NaIO4) in a solid and stir.React at room temperature for 20 min,After the reaction, 900 ml of chloroform was added.Separate the organic phase, use water,Washed twice with saturated sodium bicarbonate in 900 ml.Wash once with 900 ml of saturated sodium chloride solution, dry over anhydrous sodium sulfate, and filter.The mixture was concentrated to dryness under reduced pressure to give Compound II 49.3 g (yield 46.5 / 50 = 93.0%).
References:
CN105175391,2018,B Location in patent:Paragraph 0019; 0020; 0023; 0024; 0025
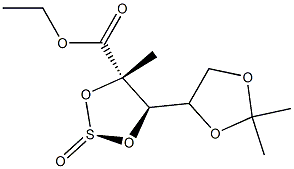
1020063-91-5
1 suppliers
inquiry

879551-01-6
12 suppliers
inquiry
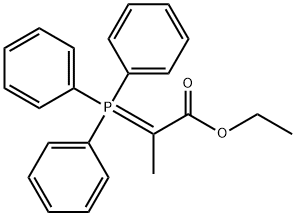
5717-37-3
452 suppliers
$6.00/5g

879551-01-6
12 suppliers
inquiry
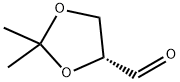
15186-48-8
322 suppliers
$8.00/5g

879551-01-6
12 suppliers
inquiry
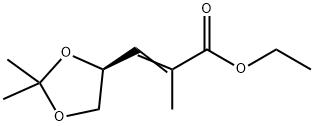
130606-67-6
5 suppliers
inquiry

879551-01-6
12 suppliers
inquiry