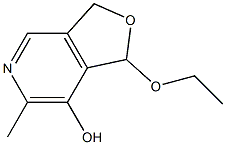
Furo[3,4-c]pyridin-7-ol, 1-ethoxy-1,3-dihydro-6-methyl- synthesis
- Product Name:Furo[3,4-c]pyridin-7-ol, 1-ethoxy-1,3-dihydro-6-methyl-
- CAS Number:880-91-1
- Molecular formula:C10H13NO3
- Molecular Weight:195.2151
Yield:880-91-1 81%
Reaction Conditions:
with phenol for 2 h;Reflux;Reagent/catalyst;
Steps:
1-Ethoxy-6-methyl-1,3-dihydrofuro[3,4-c]pyridin-7-ol (3).
General procedure: A mixture of 0.72 g of pyridoxal and 0.26 g of acetic acid in 20 mL of anhydrous ethanol was refluxed for 2 h. After cooling the solvent was removed. Anhydrous diethyl ether (20 mL) was added to the residue, the precipitate formed was separated and dried. Yield 0.58 g (69%), mp 118-121°C [4]. 1H NMR spectrum (DMSO-d6), δ, ppm: 1.13 t (3H, CH3, 2JHH = 7.1 Hz), 2.37 s (3H, CH3), 3.65 m (2H, OCH2), 4.93 d (1H, OCH2, 2JHH = 12.9 Hz), 5.03 d (1H, OCH2, 2JHH = 12.9 Hz), 6.27 s (1H, CH), 7.93 s (1H, CHAr), 9.66 s (1H, OH).
References:
Bagautdinova, R. Kh.;Kibardina;Trifonov;Pudovik;Pudovik;Burilov [Russian Journal of General Chemistry,2017,vol. 87,# 9,p. 2097 - 2099][Zh. Obshch. Khim.,2017,vol. 87,# 9,p. 1542 - 1544]
20905-66-2
1 suppliers
inquiry
![Furo[3,4-c]pyridin-7-ol, 1-ethoxy-1,3-dihydro-6-methyl-](/CAS/20180529/GIF/880-91-1.gif)
880-91-1
0 suppliers
inquiry
66-72-8
39 suppliers
inquiry
![Furo[3,4-c]pyridin-7-ol, 1-ethoxy-1,3-dihydro-6-methyl-](/CAS/20180529/GIF/880-91-1.gif)
880-91-1
0 suppliers
inquiry
![Furo[3,4-c]pyridin-1-ol, 1,3-dihydro-6-methyl-7-(phenylmethoxy)-](/CAS/20210305/GIF/14301-54-3.gif)
14301-54-3
0 suppliers
inquiry
![Furo[3,4-c]pyridin-7-ol, 1-ethoxy-1,3-dihydro-6-methyl-](/CAS/20180529/GIF/880-91-1.gif)
880-91-1
0 suppliers
inquiry

![Furo[3,4-c]pyridine-1,7-diol, 1,3-dihydro-6-methyl-, hydrochloride (1:1)](/CAS/20211123/GIF/21634-26-4.gif)