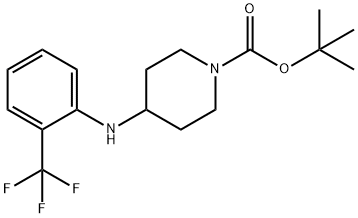
1-BOC-4-[(2-TRIFLUOROMETHYLPHENYL)AMINO]-PIPERIDINE synthesis
- Product Name:1-BOC-4-[(2-TRIFLUOROMETHYLPHENYL)AMINO]-PIPERIDINE
- CAS Number:881391-34-0
- Molecular formula:C17H23F3N2O2
- Molecular Weight:344.37
Yield:881391-34-0 95%
Reaction Conditions:
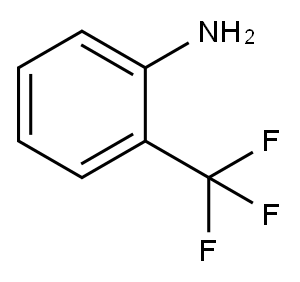
Stage #1: N-tert-butyloxycarbonylpiperidin-4-one;2-(trifluoromethyl)benzenaminewith titanium(IV) isopropylate in tetrahydrofuran at 20; for 4 h;
Stage #2: with sodium cyanoborohydride in tetrahydrofuran at 20;
Steps:
1.A
PREPARATION 1; SYNTHESIS OF PIPERIDIN^-YL-(Z-TRIFLUOROMETHYLPHENYL)AMINEA.; Titanium isopropoxide (2.5 mL, 8.46 mmol) was added to a solution of 2-aminobenzotrifluoride (0.6 mL, 4.82 mmol) and 1-Boc-4-piperidone (1.05 g, 5.26 mmol)) in THF (3 mL), the resulting mixture was stirred at ambient temperature for 4 hours, then sodium cyanoborohydride (0.800 g, 12.73 mmol) was added. The stirring was continued overnight. Aqueous sodium hydroxide (2.0 mL, 1.0 M) was added and the mixture was stirred for another 15 minutes. The reaction mixture was diluted with ethyl acetate (250 mL), washed with water, and brine, dried over anhydrous Na2SO4, and concentrated. The residue was purified by flash chromatography to afford 4-(2- trifluoromethylphenylamino)piperidine-1-carboxylic acid te/t-butyl ester (1.58 g, 95%). MS (ES+) m/z 345.1 (M+1).
References:
WO2006/34338,2006,A1 Location in patent:Page/Page column 43