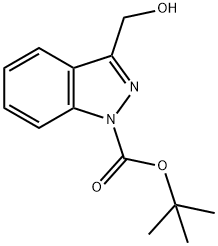
1H-Indazole-1-carboxylic acid, 3-(hydroxyMethyl)-, 1,1-diMethylethyl ester synthesis
- Product Name:1H-Indazole-1-carboxylic acid, 3-(hydroxyMethyl)-, 1,1-diMethylethyl ester
- CAS Number:882188-87-6
- Molecular formula:C13H16N2O3
- Molecular Weight:248.28

24424-99-5
834 suppliers
$13.50/25G

64132-13-4
93 suppliers
$17.00/250mg

882188-87-6
19 suppliers
$345.00/0.5 G
Yield: 84%
Reaction Conditions:
with dmap;sodium hydrogencarbonate in dichloromethane at 20; for 12 h;
Steps:
1A.C; 1B.C Step C.
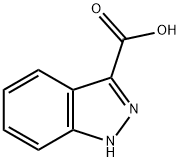
Sodium hydrocarbonate (16.6 g, 0.197 mol, 2.66 eq), DMAP (0.15 g, 1.23 mmol, 0.017 eq) were added to solution of 3 (11 g, 0.074 mol, 1 eq) in 240 mL of CH2Cl2. To this mixture, Boc2O (16.6 g, 0.076 mol, 1.03 eq) in 25 ml of CH2Cl2 was added dropwise at room temperature.11 g of compound 3 was dissolved in 240 mL of dry DCM. The reaction mixture was stirred for 12 hours, washed with water (300 mL x 3) and dried over sodium sulphate. The mixture was filtered and the filtrate was evaporated to dryness. The residue was purified by silica-gel flash chromatography (CH2Cl2/MeOH = 98:2) to give 15.5 g of the product 4 (84% yield).1H NMR (400 MHz, CDCl3) d 8.09 (d, J = 8.5 Hz, 1H), 7.81 (d, J = 8.0 Hz, 1H), 7.50 (t, J = 7.8 Hz, 1H), 7.32 (t, J = 7.5 Hz, 1H), 4.75 (s, 2H), 1.68 (s, 9H). 13C NMR (126 MHz, CDCl3) d 148.91 (s), 147.18 (s), 140.72 (s), 129.24 (s), 124.05 (s), 123.71 (s), 120.66 (s), 114.88 (s), 85.28 (s), 28.16 (s), 22.87 (s).
References:
IRON HORSE THERAPEUTICS, INC.;SMITH, Christopher Ronald;CHAPMAN, Justin WO2019/213620, 2019, A1 Location in patent:Paragraph 0632-0633; 0636
4498-67-3
469 suppliers
$6.00/5g

882188-87-6
19 suppliers
$345.00/0.5 G

43120-28-1
161 suppliers
$10.00/1g

882188-87-6
19 suppliers
$345.00/0.5 G