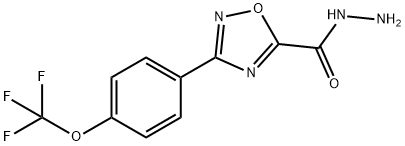
3-(4-(trifluoromethoxy)phenyl)-1,2,4-oxadiazole-5-carbohydrazide synthesis
- Product Name:3-(4-(trifluoromethoxy)phenyl)-1,2,4-oxadiazole-5-carbohydrazide
- CAS Number:883028-82-8
- Molecular formula:C10H7F3N4O3
- Molecular Weight:288.18
1258269-03-2
13 suppliers
inquiry

883028-82-8
16 suppliers
inquiry
Yield:883028-82-8 92%
Reaction Conditions:
with hydrazine in ethanol;water at 20; for 16 h;
Steps:
Step 2: synthesis of 3-(4-(trifluoromethoxy)phenyl)-1,2,4-oxadiazole -5-carbohydrazide
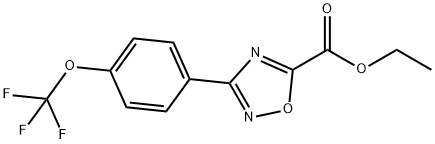
To a solution of ethyl 3-(4-(trifluoromethoxy)phenyl)-1,2,4-oxadiazole-5-carboxylate (80.0 g, 265 mmol) in EtOH (800 ml) was added NH2NH2.H2O (85%, 76.0 ml, 1,325 mmol). The reaction mixture was stirred at RT for 16 h.The desired compound precipitated from the reaction mixture and was filteredand washed with EtOH (200 ml) to produce 3-(4-(trifluoromethoxy)phenyl)-1,2,4-oxadiazole-5-carbohydrazide (70.2 g, 92%) as a light yellow solid, which was used for the next step without further purification. 1H NMR (600 MHz, Methanol-d4): δ8.24 (d, J = 8.8 Hz, 2 H), 7.47 (d, J = 8.6 Hz, 2 H). 13C NMR (126 MHz, DMSO-d6):δ 166.9, 129.4, 124.7, 121.7, 119.9 (q, J = 258 Hz). 19F NMR (471 MHz, DMSO-d6):δ -56.6. HRMS (ESI+) m/z: [M + H]+ calculated for C10H8F3N4O3, 289.0543; found,289.0538.
References:
Molina, Jennifer R.;Sun, Yuting;Protopopova, Marina;Gera, Sonal;Bandi, Madhavi;Bristow, Christopher;McAfoos, Timothy;Morlacchi, Pietro;Ackroyd, Jeffrey;Agip, Ahmed-Noor A.;Al-Atrash, Gheath;Asara, John;Bardenhagen, Jennifer;Carrillo, Caroline C.;Carroll, Christopher;Chang, Edward;Ciurea, Stefan;Cross, Jason B.;Czako, Barbara;Deem, Angela;Daver, Naval;De Groot, John Frederick;Dong, Jian-Wen;Feng, Ningping;Gao, Guang;Gay, Jason;Do, Mary Geck;Greer, Jennifer;Giuliani, Virginia;Han, Jing;Han, Lina;Henry, Verlene K.;Hirst, Judy;Huang, Sha;Jiang, Yongying;Kang, Zhijun;Khor, Tin;Konoplev, Sergej;Lin, Yu-Hsi;Liu, Gang;Lodi, Alessia;Lofton, Timothy;Ma, Helen;Mahendra, Mikhila;Matre, Polina;Mullinax, Robert;Peoples, Michael;Petrocchi, Alessia;Rodriguez-Canale, Jaime;Serreli, Riccardo;Shi, Thomas;Smith, Melinda;Tabe, Yoko;Theroff, Jay;Tiziani, Stefano;Xu, Quanyun;Zhang, Qi;Muller, Florian;Depinho, Ronald A.;Toniatti, Carlo;Draetta, Giulio F.;Heffernan, Timothy P.;Konopleva, Marina;Jones, Philip;Di Francesco, M. Emilia;Marszalek, Joseph R. [Nature Medicine,2018,vol. 24,# 7,p. 1036 - 1046]