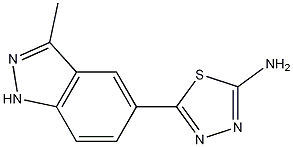
5-(3-METHYL-1H-INDAZOL-5-YL)-1,3,4-THIADIAZOL-2-AMINE synthesis
- Product Name:5-(3-METHYL-1H-INDAZOL-5-YL)-1,3,4-THIADIAZOL-2-AMINE
- CAS Number:885223-59-6
- Molecular formula:C10H9N5S
- Molecular Weight:231.28
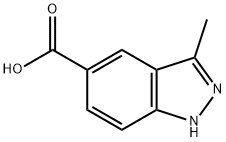
885223-58-5
38 suppliers
$45.00/10mg

79-19-6
493 suppliers
$14.00/1g

885223-59-6
8 suppliers
inquiry
Yield:885223-59-6 53%
Reaction Conditions:
with polyphosphoric acid at 90; for 24.5 h;Product distribution / selectivity;
Steps:
3.2
To a round bottom flash equipped with an overhead stir motor was added 80 g of polyphosphoric acid. The flask was heated to 90 0C and a finely ground mixture of 3 -methyl- lH-indazole-5-carboxlic acid 4e (8.0 g, 45.5 mmol) and <.-- EPO
References:
WO2006/44860,2006,A2 Location in patent:Page/Page column 23-25