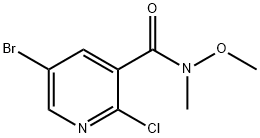
5-Bromo-2-chloro-N-methoxy-N-methylpyridine-3-carboxamide synthesis
- Product Name:5-Bromo-2-chloro-N-methoxy-N-methylpyridine-3-carboxamide
- CAS Number:885223-63-2
- Molecular formula:C8H8BrClN2O2
- Molecular Weight:279.52
29241-65-4
244 suppliers
$6.00/1g

1117-97-1
71 suppliers
inquiry

885223-63-2
31 suppliers
$55.00/250mg
Yield:885223-63-2 90%
Reaction Conditions:
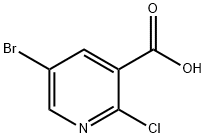
Stage #1: 5-bromo-2-chloronicotinic acidwith 1,1'-carbonyldiimidazole in dichloromethane at 20; for 1 h;
Stage #2: N,0-dimethylhydroxylamine in dichloromethane at 20;
Steps:
D-2
Example D-2: Preparation of (2S)-1 -(4-(1 -isopropyl-3-(3-methyl-1 H-pyrazolo[3,4-b]pyridin-5-yl)-1 H- pyrazol-4-yi)pyrimidin-2-ylamino)propan-2-ol
References:
WO2009/16460,2009,A2 Location in patent:Page/Page column 102-104

6638-79-5
559 suppliers
$6.00/25g

29241-65-4
244 suppliers
$6.00/1g

885223-63-2
31 suppliers
$55.00/250mg

6638-79-5
559 suppliers
$6.00/25g

78686-86-9
23 suppliers
$106.04/250mgs:

885223-63-2
31 suppliers
$55.00/250mg