
3-(2-CARBOXY-ETHYL)-MORPHOLINE-4-CARBOXYLIC ACID TERT-BUTYL ESTER synthesis
- Product Name:3-(2-CARBOXY-ETHYL)-MORPHOLINE-4-CARBOXYLIC ACID TERT-BUTYL ESTER
- CAS Number:885274-05-5
- Molecular formula:C12H21NO5
- Molecular Weight:259.3
2033-24-1
659 suppliers
$6.00/25g

833474-06-9
62 suppliers
$137.00/100mg

885274-05-5
14 suppliers
inquiry
Yield:885274-05-5 40%
Reaction Conditions:
with formic acid;triethylamine at 0 - 100; for 5 h;Cooling with ice;
Steps:
18.A Step A: 3-(4-(tert-butoxycarbonyl)morpholin-3-yl)propanoic acid
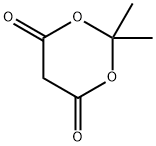
[00220] Step A: 3-(4-(tert-butoxycarbonyl)morpholin-3-yl)propanoic acidA mixture of formic acid (2.26 mL, 58.9 mmol) and triethylamine (3.36 mL, 24.11 mmol) was stirred for 5 minutes in an ice bath. To the mixture were added lert-butyl 3-formylmorpholine-4-carboxylate (0.47 g, 2.2 mmol) and Meldrum’s acid (0.32 g, 2.2 mmol) in turn at 0 C. The reaction mixture was heated at 100 °C for 5 hours, and cooled down in an ice bath. To the resulting mixture was added aqueous NaOH soluiton (2 mol/L, 40 mL). The aqueous layer was extracted with EtOAc (50 mL x 3) and the organic layer was discarded. To the aqueous layer was added EtOAc (80 mL), and the mixture was adjusted to pH 4-5 with MCI (1 mol/L) under stirring. The aqueous layer was extracted with EtOAc (80 mL). The organic layer was dried over anhydrous Na2SO4, then filtered and the filtrate was concentrated in vacuo to give the title compound as a white solid (0.23 g, 40%). The compound was characterized by the following spectroscopic data:MS-ESI: (ESI, pos.ion) m/z: 204.1 [M+1-56j1H NMR (400 MHz, CDCI3): 4.13-4.10 (m, 1H), 3.84-3.81 (m, 211), 3.80-3.74 (m, IH),3.71-3.41 (m, 211), 3.12-3.10 (m, IH), 2.38-2.35 (m, 2H), 2.25-2.21 (m, IH), 1.90-1.85 (m,111), 1.46 (s, 9H).
References:
WO2014/29193,2014,A1 Location in patent:Paragraph 00220