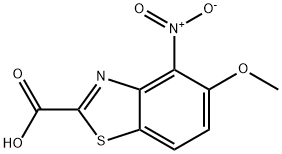
5-methoxy-4-nitrobenzo[d]thiazole-2-carboxylic acid synthesis
- Product Name:5-methoxy-4-nitrobenzo[d]thiazole-2-carboxylic acid
- CAS Number:886745-59-1
- Molecular formula:C9H6N2O5S
- Molecular Weight:254.22
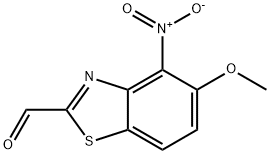
886745-60-4
0 suppliers
inquiry
![5-methoxy-4-nitrobenzo[d]thiazole-2-carboxylic acid](/CAS/GIF/886745-59-1.gif)
886745-59-1
18 suppliers
inquiry
Yield:886745-59-1 64%
Reaction Conditions:
Stage #1: 5-methoxy-4-nitrobenzothiazole-2-carboxaldehydewith sodium chlorite;disodium hydrogenphosphate;2-methyl-but-2-ene in water;tert-butyl alcohol at 20; for 18 h;
Stage #2: with hydrogenchloride in water;
Steps:
25.1.2
A solution of 18 g of sodium chlorite and 18 g of sodium hydrogen phosphate in 180 ml of water is added dropwise to the carbaldehyde residue taken up in 420 ml of tert-butanol and 100 ml of 2-methyl-but-2-ene. The reaction mixture is maintained under stirring for 18 hours at ambient temperature, then the insoluble part is filtered, taken up in water and the aqueous solution obtained is acidified by a 1M solution of hydrochloric acid. The precipitate obtained is filtered and washed with water. The acid is obtained in the form of a beige powder. (m=3.12 g; yield=64%). Melting point: 140-142° C.NMR 1H (DMSO d6, 400 MHz, δ): 8.45-8.43 (d, 1H, arom H); 7.74-7.71 (d, 1H, arom H); 3.99 (s, 3H, CH3).MS-LC: MH+=254.99; r.t.=8.20 min.
References:
US2009/275624,2009,A1 Location in patent:Page/Page column 19