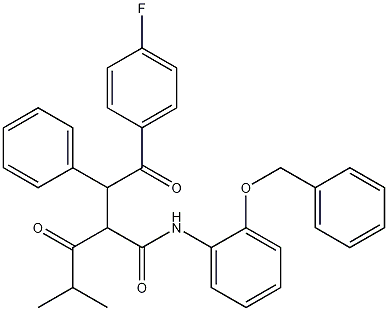
2-[2-(4-Fluorophenyl)-2-oxo-1-phenyl-ethyl]-4-methyl-3-oxo-pentanoic Acid, (2-Benzyloxy-phenyl)-amide synthesis
- Product Name:2-[2-(4-Fluorophenyl)-2-oxo-1-phenyl-ethyl]-4-methyl-3-oxo-pentanoic Acid, (2-Benzyloxy-phenyl)-amide
- CAS Number:887355-33-1
- Molecular formula:C33H30FNO4
- Molecular Weight:523.594
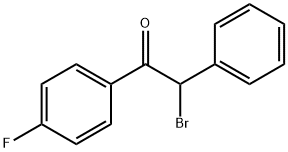
88675-31-4
71 suppliers
$25.00/250mg

265989-31-9
15 suppliers
inquiry
![2-[2-(4-Fluorophenyl)-2-oxo-1-phenyl-ethyl]-4-methyl-3-oxo-pentanoic Acid, (2-Benzyloxy-phenyl)-amide](/CAS2/GIF/887355-33-1.gif)
887355-33-1
8 suppliers
$739.20/10MG
Yield:887355-33-1 55 g
Reaction Conditions:
with potassium carbonate in tert-butyl methyl ether;isopropyl alcohol at 5 - 45; for 13.58 h;
Steps:
5 Example-5: Preparation of 4-Methyl-3-oxo-N-(2-benzyloxyphenyl)-2- Li -phenyl-2-(4- fluorophenyl)-2-oxo ethylipentanamide (Formula-9)
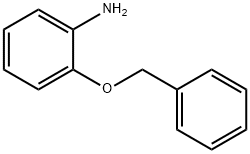
A mixture of N-(2-(benzyloxy)phenyl)-4-methyl-3-oxopentanamide (100 gms) and isopropanol (500 ml) were stirred for 15 minutes at 25-30°C. Cooled the reaction mixture to 5-10°C. Potassium carbonate (88.6 gms) was added to the reaction mixture at 25-30°C. Isopropanol (580 ml) was added to 2-bromo- 1 -(4-fluorophenyl)-2-phenylethanone (75.4 gms) at 25-30°C and stirred for 15 minutes at the same temperature. Heated the reactionmixture to 45-50°C and stirred for 20 minutes at the same temperature. Cooled the reaction mixture to 10-1 5°C and slowly added to the above reaction mixture at the same temperature. Stirred the reaction mixture for 12 hours at 10-15°C. Isopropanol (300 ml) was added to the reaction mixture at 10-15°C and stirred for 1 hour at the same temperature. Filtered the undissolved material and washed with isopropanol. Distilled off the solvent completely fromthe filtrate under reduced pressure and cooled to 25-30°C. Dichloromethane was added to the obtained compound at 25-30°C and stirred for 20 minutes at the same temperature. Purified water (200 ml) was added to the reaction mixture at 25-30°C and stirred for 20 minutes at the same temperature. Both the organic and aqueous layers were separated and organic layer was washed with purified water. The organic layer was dried over sodium sulfate. Distilled off thesolvent completely from the organic layer under reduced pressure. Methyl tertiary butyl ether (100 ml) was added to the obtained compound at 40-45°C and stirred for 1.5 minutes at the same temperature. Distilled off the solvent completely from the organic layer under reduced pressure. Methyl tertiary butyl ether (200 ml) was added to the obtained compound and stirred for 30 minutes at the same temperature. Cooled the reaction mixture to 0-5°C andseeded with compound of formula-9 at the same temperature. Stirred the reaction mixture for3 hours at 0-5°C. Filtered the precipitated solid, washed with chilled methyl tertiary butyl ether and dried to get the title compound. Yield: 55 gms; Melting point: 102-104°C.Example-6: Preparation of (4R-cis)-l ,l-dimethylethyl-6- [2- [2-(4-fluorophenyl)-5-(1- methylethyl)-3-phenyl-4-[(4-benzyloxyphenyl)carbonyl] -1H-pyrrol-yl-] ethyl] -2,2-dimethyl-l,3-dioxane-4-acetate (Formula-li)
References:
WO2016/142954,2016,A2 Location in patent:Page/Page column 14; 17
20012-63-9
152 suppliers
$19.00/1g
![2-[2-(4-Fluorophenyl)-2-oxo-1-phenyl-ethyl]-4-methyl-3-oxo-pentanoic Acid, (2-Benzyloxy-phenyl)-amide](/CAS2/GIF/887355-33-1.gif)
887355-33-1
8 suppliers
$739.20/10MG
4560-41-2
48 suppliers
inquiry
![2-[2-(4-Fluorophenyl)-2-oxo-1-phenyl-ethyl]-4-methyl-3-oxo-pentanoic Acid, (2-Benzyloxy-phenyl)-amide](/CAS2/GIF/887355-33-1.gif)
887355-33-1
8 suppliers
$739.20/10MG

265989-31-9
15 suppliers
inquiry
![2-[2-(4-Fluorophenyl)-2-oxo-1-phenyl-ethyl]-4-methyl-3-oxo-pentanoic Acid, (2-Benzyloxy-phenyl)-amide](/CAS2/GIF/887355-33-1.gif)
887355-33-1
8 suppliers
$739.20/10MG

459-57-4
595 suppliers
$6.00/25g
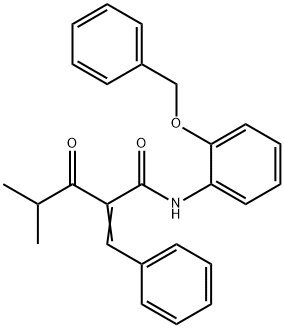
163217-77-4
13 suppliers
inquiry
![2-[2-(4-Fluorophenyl)-2-oxo-1-phenyl-ethyl]-4-methyl-3-oxo-pentanoic Acid, (2-Benzyloxy-phenyl)-amide](/CAS2/GIF/887355-33-1.gif)
887355-33-1
8 suppliers
$739.20/10MG