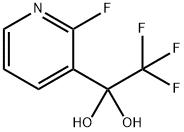
3-PyridineMethanol,2-fluoro-α-(nitroMethyl)-α-(trifluoroMethyl)- synthesis
- Product Name:3-PyridineMethanol,2-fluoro-α-(nitroMethyl)-α-(trifluoroMethyl)-
- CAS Number:892414-45-8
- Molecular formula:C8H6F4N2O3
- Molecular Weight:254.14
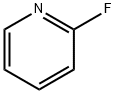
372-48-5
368 suppliers
$5.00/5g

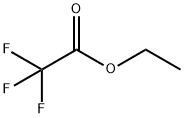
75-52-5
4 suppliers
$35.28/100g
383-63-1
473 suppliers
$10.00/25g

892414-45-8
8 suppliers
inquiry
Yield:892414-45-8 85%
Reaction Conditions:
Stage #1: 2-fluoropyridinewith lithium diisopropyl amide in tetrahydrofuran at -75; for 4 h;
Stage #2: ethyl trifluoroacetate, in tetrahydrofuran at -45 - 20;
Stage #3: nitromethane in tetrahydrofuran;
Steps:
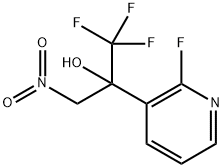
1.1 Step 1 : 1, 1, 1 -trifluoro-2-(2-fluoropyridin-3-yl)-3-nitropropan-2-ol
To a solution of freshly prepared lithium diisopropylamide (42.5 g, 0.55 mol) in tetrahydrofuran (1200mL) at -75 °C was added 2-fluoropyridine (45 g, 0.46 mol) and the mixture was stirred for 4 h at this temperature. To the resulting stirred suspension, ethyl trifluoroacetate (91.4 g, 0.64 mol) was added while ensuring the temperature did not rise above -45 °C. The reaction mixture was warmed to RT., nitromethane (56.1 g, 0.92 mol) was added, and the reaction was stirred overnight. The solution was poured into 2N aqueous hydrochloric acid (6 L), and the mixture was extracted with ethyl acetate (3 x 500mL). The combined organic layers were washed with brine, dried over sodium sulfate and concentrated to dryness in vacuo. The resulting residue was triturated with petroleum ether, and the product was collected by suction filtration to give l, l,l-trifluoro-2-(2-fluoropyridin-3-yl)-3-nitropropan-2-ol (100 g, 85 %): NMR (400 MHz, DMSO-d6): δ 8.22-8.35 (m, 3H), 7.47-7.51 (m, 1H), 5.65 (d, J = 13.2 Hz, 1H), 5.11 (d, J = 13.2 Hz, 1H
References:
WO2014/111496,2014,A1 Location in patent:Page/Page column 104

372-48-5
368 suppliers
$5.00/5g

892414-45-8
8 suppliers
inquiry